��Ŀ����
18��þ������ɫ��������ʵ���ҵ�þ�����泣����һ�ֻҺ�ɫ���ʣ���ij�о���ѧϰС��Ϊ��̽��þ������Һ�ɫ���ʵ���ɣ����������¶���ʵ�飺
����ɰֽС�ĵĴ�ĥþ���������������ʵķ�ĩ״��Ʒ��
��ȡ��Ʒ����������ˮ�������飬���ָ÷�ĩ������ˮ��
����ȡ��Ʒ���������Ⱥ���ˮ�������ɣ�
����ȡ��Ʒ����������ϡ���ᣬ��������ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǣ�
��1����ͬѧ��������֪ʶ����Ԥ�⣺þ������ĻҺ�ɫ������̼����þ������ʵ��ڣ�����ţ������Ʒ���ͬѧ��Ԥ�⣮
��2����ͬѧԤ��þ������ĻҺ�ɫ�����Ǽ�ʽ̼��þ���������������ϻ��������Ϣ��
��ʽ̼��þMgx��OH��y��CO3��z��x��y��zΪ��������������ˮ��������ϡ���ᣬ���ȷֽ���������þ��������̼��ˮ��
��д����ʽ̼��þMgx��OH��y��CO3��z����ϡ��������ӷ���ʽ������ĸx��y��z��ƽ����Mgx��OH��y��CO3��z+2x H+=x Mg2++z CO2��+��y+z��H2O��
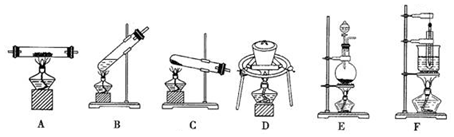
��Ϊȷ���ûҺ�ɫ���ʵ���ɣ�С���Ա�������ͼ��ʾ��ʵ��װ�ã��г�����δ���������ж���̽�������������Ӻ��������������������ú�װ����Ӧ������ҩƷ��

��1��A��U�ι�����ʢ�ŵ�ҩƷ�������dz�ȥ����װ�õĿ����������е�ˮ�����Ͷ�����̼��
��2����ͬѧ������ڳ�����Ӧװ�õij�ʼ����ǰ����Ҫ�ȴ���K1����һ�������������һ���������Ŀ��������װ����ԭ����ˮ�����Ͷ�����̼��ʵ���Ӱ�죮
��3��ʵ�鿪ʼ����ǰ����Ҫ�ֱ����B����������װ�����Ʒ������װ��CD�ij�ʼ����������ţ�
��4��װ��B�еķ�Ӧ��ȫ����Ҫ����K1���ٹ���һ���������Ȼ���ٷֱ������Ӧװ�õ�����������
��5����ͬѧ�����Ҫ��E�����иĽ���������������ͬѧ����ȷ�Ľ�����������һ��װ�м�ʯ�ң��������ƣ��ĸ���ܣ�
��6�������Ľ����ã�װ��C��D��ʵ��ǰ������طֱ�Ϊ1.8g��4.4g����֪��Ʒ�е�������ʵ���в���ֽ⣬����ȷ������Ʒ�м�ʽ̼��þMgx��OH��y��CO3��z�����Ϊx��y��z=2��2��1������������ȣ���
���� ��1��̼����þ����ˮ��
��2����ʽ̼��þMgx��OH��y��CO3��z����ϡ������������þ��������̼��ˮ�����ԭ���غ���ƽ��д��
��1�����ü�ʯ�ҳ�ȥ������ˮ�����Ͷ�����̼��
��2���ڳ�����Ӧװ�õij�ʼ����ǰ����Ҫ�ȴ���K1����һ������������ó�ȥˮ�����Ͷ�����̼�Ŀ�����װ���ڿ����ž���������Ųⶨ�����
��3��ʵ��ⶨ�ֽ���������ɶ�����̼��ˮ����������Ҫ�ֱ������Ӧװ�÷�Ӧǰ�����շ�Ӧ���������
��4��װ��B�еķ�Ӧ��ȫ���ó�ȥˮ�кͶ�����̼�Ŀ��������ɵ�ˮ�кͶ�����̼�������װ����ȫ���գ��ⶨ�����ȷ��
��5�����������ˮ�����Ͷ�����̼����װ��D��Ҫ��E��������һ��ʢ��ʯ�ҵĸ���ܣ�
��6�������Ľ����ã�װ��C��D��ʵ��ǰ������طֱ�Ϊ1.8g��4.4g���ֱ�Ϊˮ�Ͷ�����̼����������������ʵ����ʵ���������Ԫ���غ�����ʽ̼��þ��y��z�����Ԫ�ػ��ϼ۴�����Ϊ0����x��
��� �⣺��1��ȡ��Ʒ����������ˮ�������飬���ָ÷�ĩ������ˮ��þ������ĻҺ�ɫ��������̼����þ��������ˮ�����Ԣڿ����Ʒ���ͬѧ��Ԥ�⣬
�ʴ�Ϊ���ڣ�
��2����ʽ̼��þMgx��OH��y��CO3��z����ϡ������������þ��������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��Mgx��OH��y��CO3��z+2x H+=x Mg2++z CO2��+��y+z��H2O��
�ʴ�Ϊ��Mgx��OH��y��CO3��z+2x H+=x Mg2++z CO2��+��y+z��H2O��
��1��A��U�ι�����ʢ�ŵ�ҩƷ�Ǽ�ʯ�ң������dz�ȥ����װ�õĿ����������е�ˮ�����Ͷ�����̼��
�ʴ�Ϊ����ȥ����װ�õĿ����������е�ˮ�����Ͷ�����̼��
��2����ͬѧ������ڳ�����Ӧװ�õij�ʼ����ǰ����Ҫ�ȴ���K1����һ�������������һ���������Ŀ��������װ����ԭ����ˮ�����Ͷ�����̼��ʵ���Ӱ�죬
�ʴ�Ϊ������װ����ԭ����ˮ�����Ͷ�����̼��ʵ���Ӱ�죻
��3��ʵ�鿪ʼ����ǰ����Ҫ�ֱ����B����������װ�����Ʒ������װ��CD���������÷�Ӧǰ�������仯��������ˮ�Ͷ�����̼��������
�ʴ�Ϊ��C��D��
��4��װ��B�еķ�Ӧ��ȫ���ó�ȥˮ�кͶ�����̼�Ŀ��������ɵ�ˮ�кͶ�����̼�������װ����ȫ���գ�����Ϊ������K1���ٹ���һ���������
�ʴ�Ϊ������K1���ٹ���һ���������
��5��Ϊ���������ˮ�����Ͷ�����̼����װ��D��Ҫ��E��������һ��ʢ��ʯ�ҵĸ����
�ʴ�Ϊ������һ��װ�м�ʯ�ң��������ƣ��ĸ���ܣ�
��6��װ��C��D��ʵ��ǰ������طֱ�Ϊ1.8g��4.4g������ˮ���ʵ���=$\frac{1.8g}{18g/mol}$=0.1mol�����ɶ�����̼���ʵ���=$\frac{4.4g}{44g/mol}$=0.1mol��Ԫ���غ�õ�y=0.2mol��z=0.1mol�����Ԫ�ػ��ϼ۴�����Ϊ0����x=2������ȷ������Ʒ�м�ʽ̼��þMgx��OH��y��CO3��z�����Ϊx��y��z=2��2��1���ʴ�Ϊ��2��2��1��
���� ���⿼��ѧ����ʵ��ԭ�����⡢������Ƶ����ۡ�ʵ��װ�õ����⡢������ɵIJⶨ�ȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺ����ã���Ҫѧ��������ʵ�Ļ������ۺ����÷�����������������


��1��MgCO3����ϡ��������ӷ���ʽ��MgCO3+2H+�TMg2++CO2��+H2O��
��2������H2O2��Һ��Ŀ����H2O2+2Fe2++2H+�T2Fe3++2H2O�������ӷ���ʽ��ʾ����
��3����֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Mg2+ | Fe2+ | Fe3+ |
| ��ʼ���� | 9.1 | 7.6 | 1.9 |
| ��ȫ���� | 11.1 | 9.7 | 3.2 |
��4�������ˡ�������Һ�д��ڴ�������������Mg2+��NH4+��
��5�����ᾧ��������������Ũ����Һ��ʹ�õ�����������̨���ƾ��ƺ�������������
���������գ�
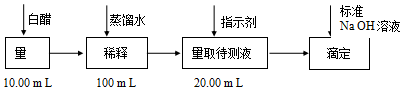
��1��ϡ�Ͱ״�ʱ��Ҫ���������ձ�������������ͷ�ιܡ�100mL����ƿ��
��2��Ӧѡ�÷�̪��Ϊָʾ�����ﵽ�ζ��յ�ʱ��ָʾ������ɫ��Ϊdz��ɫ��
��3��ijͬѧһ������������ʵ�飮����������Ƶ�ʵ�����ݼ�¼����������A�ǵζ�ǰ������B�ǵζ��������
| ʵ����� | ϡ�ͺ�״� �����mL�� | ��NaOH��Һ | ||
| A | B | ���������mL�� | ||
| 1 | 20.00 | 22.05 | ||
| 2 | 20.00 | 21.34 | ||
| 3 | 20.00 | 21.30 | ||
�����ϡ�ͺ�״�Ũ��0.0594mol/L�����ʳ���ϣ�ѡ����ϡ����������ϡ������ұ���
��NaOH��Һͨ�����²�������������500mLŨ��ԼΪ0.1mol/L��NaOH��Һ��
����KHC8H4O4����Һȷ�ⶨ��NaOH��Һ��Ũ�ȣ�
��4�����������NaOH�������ڴ��ձ��У�����500mL����ˮ�������ܽ⣬�����Ʋ�����п���
�����������������
��5��NaOH����Һ��Ũ����ͨ���ⶨ������ֱ�����Ƶ�ԭ����NaOH�����ڳ���ʱ�������տ����е�ˮ��CO2��ʹ�������õ���ҺŨ�ȵ���Ԥ��Ũ�ȣ�����ʵ����
| A�� |  ͼ��ʾ0.1molMgCl2•6H2O�ڿ����г�ּ���ʱ����������ʱ��ı仯 | |
| B�� |  ͼ��ʾ��0.1000 mol•L��lNaOH��Һ�ζ�25.00 mLCH3COOH�ĵζ����ߣ���c��CH3COOH��=0.0800 mol•L��1 | |
| C�� |  ͼ��ʾ���º��������£�2NO2��g��?N2O4��g���У������ʵ�Ũ��������������֮��Ĺ�ϵ�����н���A��Ӧ��״̬Ϊ��ѧƽ��״̬ | |
| D�� |  ͼ��ʾ�����£�ϡ��HA��HB�������ϡ��Һʱ����ҺpH���ˮ���ı仯�������£�NaA��Һ��pHС��ͬŨ�ȵ�NaB��Һ��pH |
| ѡ�� | �������ʵ | ���� |
| A | ����п�̵�ر���ͨп�̵�����ܺ� | п�ڼ��Խ����б������ɸ���̬ |
| B | ʩ��ʱ����ľ�ң���Ч�ɷ�ΪK2CO3����������ε��ʻ��ʹ�� | ���Ƿ�Ӧ���ɰ����ή�ͷ�Ч |
| C | ����FeCl2��Һʱ������������ | ����Fe2+��ˮ�� |
| D | ��ˮ��ľͷ�������磬��ľͷ��ˮ��ʪ��ȴ���Ե��� | ˮ��ľͷ��ijЩ�ɷַ�����ѧ��Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��״���£�5.6 LNO��5.6LO2��Ϻ�ķ�������Ϊ0.5 NA | |
| B�� | 1mol������Ӻ���8NA�����ۼ� | |
| C�� | 58.5 g�Ȼ��ƹ����к���NA���Ȼ��Ʒ��� | |
| D�� | ��״���£�22.4L��������������������Һ��Ӧת�Ƶĵ�����ΪNA |
I��ClO2���Ʊ�
��֪����ǿ������Һ����SO2��ԭNaC1O3���Ʊ�ClO2��

��1����Ӧ������Բ����ƿ�ڻῴ����Һ����һ�ְ�ɫ����������Ϊ��֤�������ʳ����õ�ʵ�����������Ϊ����ʵ����ɡ�Բ����ƿ��ȴ����Ӧ��Ļ������������ʢ������ˮ���Թ���������ܽ⣬��Һ���������ɫ����Ϊ����ͭ��
��2��װ��B�з�Ӧ�����ӷ���ʽΪSO2+2ClO3-=2ClO2+SO42-��
��3�����Ʊ����ռ�ClO2��ѡ����ͼ�е�װ�ã�������˳��Ϊa��g��h��b��c��e��f��d��������������Сд��ĸ��ʾ��
��4��װ��D�������������ռ�ClO2��
II��ClO2��Na2S�ķ�Ӧ
��5���������ռ�����ClO2��N2ϡ������ǿ���ȶ��ԣ�����������ϡ�ͺ��ClO2ͨ ��ͼ2��ʾװ���г�ַ�Ӧ���õ���ɫ������Һ��һ��ʱ���ͨ������ʵ��̽��I�з�Ӧ�IJ��
| �������� | ʵ������ | ���� |
| ȡ����1����Һ���Թܼ��У��μ�Ʒ����Һ�����ᣮ | Ʒ��ʼ�ղ���ɫ | ����SO2����HSO3-��SO32-������ |
| ��ȡ����1����Һ���Թ����У�����Ba��OH��2��Һ���� | �ڰ�ɫ���� | ��SO42-���� |
| �ۼ������Թ����еμ�Ba��OH��2 ��Һ�����������ã�ȡ�ϲ���Һ���Թ��У���������ϡ�����ữ����������Һ�� | �а�ɫ�������� | ��Cl-���� |