��Ŀ����
���û�ѧ֪ʶ��������������е��й����ⱸ�ܹ�ע����ش��������⣺��1����֪ˮ��������ƽ�⣺H2O?H++OH-��H��0������ʹƽ�������ƶ�����������Һ�Լ��ԣ�ѡ���� ��
A����ˮ�м���NaHSO4���� B����ˮ�м�Na2CO3����
C��������100��[����c��H+��=1×10-6 mol?L-1]D����ˮ�м���NH4Cl����
��2�������£�Ũ�Ⱦ�Ϊ0.1mol?L-1����������������Һ��pH���±���
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
A��HCN B��HClO C��CH3COOH D��H2CO3
��3��ʵ�����г���NaOH������ϴ�����ᴿ����400mL 1mol?L-1��NaOH��Һ���ձ�״����4.48LCO2ʱ��������Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
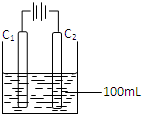
��4��ʵ�����в��ö��Ե缫ģ�ҵ�ϵ�ⱥ��ʳ��ˮ�Ĺ��̣�
��д��C1�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ ��
�ڵ�C2�缫�ϲ���112mL����״��������ʱ������������ȫ�ݳ�����Һ������䣩���ձ�����Һ��pH= ����Kw=10-14��

��2��Խ����ˮ����Σ�Խ����������ӣ���ͬŨ�ȵ�������Һ��pHԽ�����ε�ˮ��̶�Խ��Ũ����ͬ����ϡ����ͬ�ı���������Խǿ������pH�仯Խ��
��3�����������ˮ��͵���غ���������ע�������ˮ�������ģ�
��4���ٵ�ⱥ�͵��Ȼ�����Һʱ�������������ӷŵ�����������
�������������ӷŵ���������������ԭ���غ��������Ӻ����������ӵĹ�ϵ��������������Ũ�ȣ��ٸ���ˮ�����ӻ�����������Һ��������Ũ�ȣ��Ӷ��ó���Һ��pH��
����⣺��1��A����ˮ�м���NaHSO4���壬���������ܽ�����������ӣ�����ˮ���룬����Һ��C��H+����C��OH-������Һ�����ԣ��ʴ���
B����ˮ�м�Na2CO3���壬̼������ǿ����������ˮ�⣬̼������Ӻ������ӽ������̼��������ӣ��Ӷ��ٽ�ˮ���룬������Һ��C��OH-����C��H+������Һ�ʼ��ԣ�����ȷ��
C��ˮ�ĵ��������ȷ�Ӧ��������100�棬�ٽ�ˮ���룬��ҺC��OH-��=C��H+������Һ�����ԣ��ʴ���
D����ˮ�м���NH4Cl���壬�Ȼ��ˮ�⣬笠����Ӻ����������ӷ�Ӧ����һˮ�ϰ����Ӷ��ٽ�ˮ���룬������Һ��C��H+����C��OH-������Һ�����ԣ��ʴ���
��ѡB��
��2��Խ����ˮ����Σ�Խ����������ӣ���ͬŨ�ȵ�������Һ��pHԽ�����ε�ˮ��̶�Խ����������ˮ�������̼���ƣ�����������������ӵ���������CO32-��Ũ����ͬ����ϡ����ͬ�ı���������Խǿ������pH�仯Խ��HCN��HClO��CH3COOH��H2CO3����������Դ�С˳����CH3COOH��H2CO3��HClO��HCN��������Һ��pH�仯�����Ǵ��ᣬ��ѡC��
�ʴ�Ϊ��CO32-��C��
��3��400mL 1mol?L-1��NaOH�����ʵ���=1mol/L×0.4L=0.4mol����״����4.48LCO2�����ʵ���=
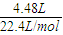 =0.2mol�������������ƺͶ�����̼��Ӧ����ʽΪCO2+2NaOH=Na2CO3+H2O����Һ��̼�������ˮ���ʹ��Һ�ʼ��ԣ���C��OH-����C��H+������Һ��������������������Դ��һ����̼�������ˮ�⣬һ����̼���������ˮ�⣬̼���������ֻ��̼�������ˮ�⣬����C��OH-����C��HCO3-����̼�������ˮ��̶ȴ���ˮ����������ӳ̶ȣ�������Һ��C��HCO3-����C��H+����
=0.2mol�������������ƺͶ�����̼��Ӧ����ʽΪCO2+2NaOH=Na2CO3+H2O����Һ��̼�������ˮ���ʹ��Һ�ʼ��ԣ���C��OH-����C��H+������Һ��������������������Դ��һ����̼�������ˮ�⣬һ����̼���������ˮ�⣬̼���������ֻ��̼�������ˮ�⣬����C��OH-����C��HCO3-����̼�������ˮ��̶ȴ���ˮ����������ӳ̶ȣ�������Һ��C��HCO3-����C��H+��������Һ�и�����Ũ�ȴ�С˳����C��Na+����C��CO32-����C��OH-����C��HCO3-����C��H+����
�ʴ�Ϊ��C��Na+����C��CO32-����C��OH-����C��HCO3-����C��H+����
��4���ٸ���ͼƬ֪��C1�缫�������������������ӷŵ������������缫��ӦʽΪ2Cl--2e-�TCl2�����ʴ�Ϊ��2Cl--2e-�TCl2����
��C2�缫�������ӷŵ��������������������ʵ���=
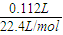 =0.005mol��
=0.005mol��H2----------2H+--------2OH-
1mol 2mol 2mol
0.005mol 0.01mol 0.01mol
������Һ�����������ӵ����ʵ���Ũ��=
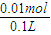 =0.1mol/L������Һ��������Ũ��=
=0.1mol/L������Һ��������Ũ��= =10-13mol/L��������Һ��pH=13��
=10-13mol/L��������Һ��pH=13���ʴ�Ϊ��13��
���������⿼��������Ũ�ȴ�С�ıȽϡ���ҺpH�ļ����֪ʶ�㣬����Ũ�ȴ�С�ıȽ���ѧϰ���ѵ㣬һ�����õ���غ㡢�����غ�������غ����������

���û�ѧ֪ʶ��������������е��й����ⱸ�ܹ�ע����ش��������⣺
��1����֪ˮ��������ƽ�⣺H2O![]() H++OH- ��H>0������ʹƽ�������ƶ�����������Һ�Լ��ԣ�ѡ���� ��
H++OH- ��H>0������ʹƽ�������ƶ�����������Һ�Լ��ԣ�ѡ���� ��
A. ��ˮ�м���NaHSO4���� B. ��ˮ�м�Na2CO3����
C. ������100��[����c(H+) = 1��10-6 mol•L-1] D. ��ˮ�м���NH4Cl����
��2�������£�Ũ�Ⱦ�Ϊ0��1mol��L-1����������������Һ��pH���±���
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8��8 | 9��7 | 11��6 | 10��3 | 11��1 |
��������Һ�е������ӣ����H+������ǿ����_________�����ݱ������ݣ�Ũ�Ⱦ�Ϊ0��01mol��L��1���������������Һ�ֱ�ϡ��100����pH�仯������________�����ţ���
A��HCN B��HClO C��CH3COOH D��H2CO3
��3��ʵ�����г���NaOH������ϴ�����ᴿ����400mL 1mol��L-1��NaOH��Һ���ձ�״����4��48LCO2ʱ��������Һ�и�����Ũ���ɴ�С��˳��Ϊ________________��
 ��4��ʵ�����в��ö��Ե缫ģ�ҵ�ϵ�ⱥ��ʳ��ˮ�Ĺ��̣�
��4��ʵ�����в��ö��Ե缫ģ�ҵ�ϵ�ⱥ��ʳ��ˮ�Ĺ��̣�
��д��C1�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ ��
�ڵ�C2�缫�ϲ���112mL(��״��)����ʱ������������ȫ
�ݳ�����Һ������䣩���ձ�����Һ��pH= ����Kw=10-14��
 ���û�ѧ֪ʶ��������������е��й����ⱸ�ܹ�ע����ش��������⣺
���û�ѧ֪ʶ��������������е��й����ⱸ�ܹ�ע����ش��������⣺