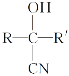МвДҝДЪИЭ
ЎҫМвДҝЎҝДіУР»ъОпКЗРДСӘ№ЬТ©өДЦчТӘіЙ·ЦЈ¬ЖдәПіЙПЯВ·ИзПВ:
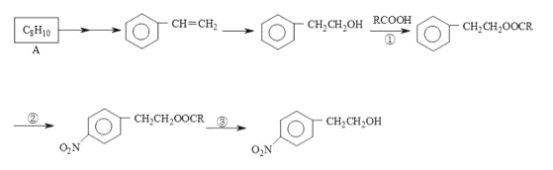
(1)A өДҪб№№јтКҪОӘ____Ј»ҙУ A ЦЖИЎұҪТТП©РиТӘҫӯ№эБҪІҪЈ¬өЪТ»ІҪКЗCl2/№вХХЈ¬ЗлРҙіцөЪ¶юІҪөД»ҜС§·ҪіМКҪ____ЎЈ
(2)·ҙУҰўЪөД·ҙУҰАаРНКЗ__________·ҙУҰЎЈ
(3)·ҙУҰўЩўЫөДДҝөДКЗ____________ ЎЈ
(4)·ҙУҰўЫөДКФјБәНМхјюКЗ_____________ ЎЈ
(5)¶ЎұҪПрҪәКЗіЈУГөД№ӨТөПрҪәЈ¬ЖдУРұҪТТП©әН 1,3-¶Ў¶юП©№ІҫЫөГөҪЈ¬ЗлРҙіцЖдҪб№№јтКҪ_______
(6)ЗлРҙіц 1,3-¶Ў¶юП©ЦЖИЎ HOCH2CH2CH2CH2OH өДУР»ъәПіЙПЯВ·ЎЈ_________________
Ўҫҙр°ёЎҝ![]()
![]() +NaOH
+NaOH![]()
 +NaCl+ H2O ИЎҙъ·ҙУҰЈЁ»тПх»Ҝ·ҙУҰЈ© ұЈ»ӨфЗ»щІ»ұ»Сх»Ҝ NaOH ИЬТәЈ¬јУИИ
+NaCl+ H2O ИЎҙъ·ҙУҰЈЁ»тПх»Ҝ·ҙУҰЈ© ұЈ»ӨфЗ»щІ»ұ»Сх»Ҝ NaOH ИЬТәЈ¬јУИИ 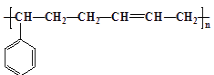 ЈЁ»т
ЈЁ»т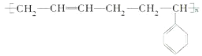 Ј© CH2=CHCH=CH2
Ј© CH2=CHCH=CH2![]() CH2BrCH=CHCH2Br
CH2BrCH=CHCH2Br![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br![]() HOCH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH
ЎҫҪвОцЎҝ
 ҫӯ№эИЎҙъ·ҙУҰәНПыИҘ·ҙУҰБҪІҪөГөҪЈ¬ҪбәПAөД·ЦЧУКҪҝЙөГAОӘұҪТТНйЈ¬Ҫб№№јтКҪОӘ
ҫӯ№эИЎҙъ·ҙУҰәНПыИҘ·ҙУҰБҪІҪөГөҪЈ¬ҪбәПAөД·ЦЧУКҪҝЙөГAОӘұҪТТНйЈ¬Ҫб№№јтКҪОӘ![]() Ј»
Ј» әНЛ®ФЪҙЯ»ҜјБЧчУГПВ·ўЙъјУіЙ·ҙУҰЈ¬ЙъіЙ
әНЛ®ФЪҙЯ»ҜјБЧчУГПВ·ўЙъјУіЙ·ҙУҰЈ¬ЙъіЙ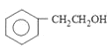 Ј¬ФЪУлУР»ъЛб·ўЙъхҘ»Ҝ·ҙУҰЙъіЙ
Ј¬ФЪУлУР»ъЛб·ўЙъхҘ»Ҝ·ҙУҰЙъіЙ![]() Ј¬
Ј¬![]() ФЪБтЛбЧчҙЯ»ҜјБМхјюПВУлПхЛб·ҙУҰЙъіЙ
ФЪБтЛбЧчҙЯ»ҜјБМхјюПВУлПхЛб·ҙУҰЙъіЙ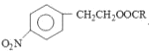 Ј¬
Ј¬ ФЪјоРФМхјюПВ·ўЙъЛ®ҪвЙъіЙ
ФЪјоРФМхјюПВ·ўЙъЛ®ҪвЙъіЙ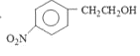 ЎЈ
ЎЈ
(1)УЙ ҫӯ№эИЎҙъ·ҙУҰәНПыИҘ·ҙУҰБҪІҪөГөҪЈ¬ҪбәПAөД·ЦЧУКҪҝЙөГAОӘұҪТТНйЈ¬Ҫб№№јтКҪОӘ
ҫӯ№эИЎҙъ·ҙУҰәНПыИҘ·ҙУҰБҪІҪөГөҪЈ¬ҪбәПAөД·ЦЧУКҪҝЙөГAОӘұҪТТНйЈ¬Ҫб№№јтКҪОӘ![]() Ј»өЪ¶юІҪЦЖИЎ
Ј»өЪ¶юІҪЦЖИЎ![]() өД»ҜС§·ҪіМКҪОӘ
өД»ҜС§·ҪіМКҪОӘ![]() +NaOH
+NaOH![]()
 +NaCl+ H2OЈ»
+NaCl+ H2OЈ»
ҙр°ёОӘЈә![]() Ј»
Ј»![]() +NaOH
+NaOH![]()
 +NaCl+ H2OЈ»
+NaCl+ H2OЈ»
(2)УРЙПКц![]() ФЪБтЛбЧчҙЯ»ҜјБМхјюПВУлПхЛб·ҙУҰЙъіЙ
ФЪБтЛбЧчҙЯ»ҜјБМхјюПВУлПхЛб·ҙУҰЙъіЙ Ј¬Пх»щҪ«ұҪ»·ЙПөДЗвФӯЧУИЎҙъЈ¬ФтОӘИЎҙъ·ҙУҰЈЁ»тПх»Ҝ·ҙУҰЈ©Ј»
Ј¬Пх»щҪ«ұҪ»·ЙПөДЗвФӯЧУИЎҙъЈ¬ФтОӘИЎҙъ·ҙУҰЈЁ»тПх»Ҝ·ҙУҰЈ©Ј»
ҙр°ёОӘЈәИЎҙъ·ҙУҰЈЁ»тПх»Ҝ·ҙУҰЈ©Ј»
(3)УЙ·ҙУҰБчіМҝЙЦӘЧоЦХТӘөГөҪә¬УРфЗ»щөД»ҜәПОпЈ¬ТтҙЛЈ¬ўЩўЫөДДҝөДКЗОӘБЛ·АЦ№·ҙУҰ№эіМЦРфЗ»щұ»Сх»ҜЎЈ
ҙр°ёОӘЈәұЈ»ӨфЗ»щІ»ұ»Сх»ҜЎЈ
(4)·ҙУҰўЫКЗхҘөДЛ®Ҫв·ҙУҰЈ¬ ФЪЗвСх»ҜДЖИЬТәәНјУИИөДМхјюПВ·ўЙъЛ®Ҫв·ҙУҰЙъіЙ
ФЪЗвСх»ҜДЖИЬТәәНјУИИөДМхјюПВ·ўЙъЛ®Ҫв·ҙУҰЙъіЙ Ј»
Ј»
ҙр°ёОӘЈәNaOH ИЬТәЈ¬јУИИЈ»
(5)¶ЎұҪПрҪәУЙұҪТТП©әН1Ј¬3-¶Ў¶юП©№ІҫЫөГөҪЈ¬Ҫб№№јтКҪ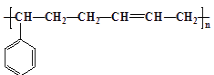 »т
»т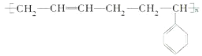
ҙр°ёОӘЈә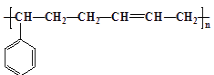 ЈЁ»т
ЈЁ»т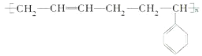 Ј©Ј»
Ј©Ј»
(6) CH2=CHCH=CH2![]() CH2BrCH=CHCH2Br
CH2BrCH=CHCH2Br![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br![]() HOCH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH
ҙр°ёОӘЈәCH2=CHCH=CH2![]() CH2BrCH=CHCH2Br
CH2BrCH=CHCH2Br![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br![]() HOCH2CH2CH2CH2OHЎЈ
HOCH2CH2CH2CH2OHЎЈ

 ҝЖС§КөСй»о¶ҜІбПөБРҙр°ё
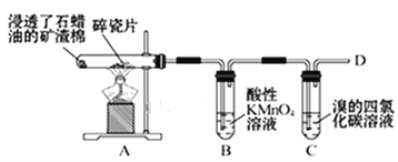
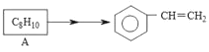
ҝЖС§КөСй»о¶ҜІбПөБРҙр°ёЎҫМвДҝЎҝАыУГИзНјЛщКҫЧ°ЦГҪшРРПВБРКөСйЈ¬ДЬөГөҪПаУҰКөСйҪбВЫөДКЗЈЁ Ј©

СЎПо | ўЩ | ўЪ | ўЫ | КөСйҪбВЫ |
A | ЕЁБтЛб | Na2SO3 | ЛбРФKMnO4ИЬТәНКЙ« | SO2УРЖҜ°ЧРФ |
B | ЕЁБтЛб | Cu | Ж·әмИЬТә | SO2ҫЯУРЖҜ°ЧРФ |
C | ПЎСОЛб | МјЛбДЖ | ВИ»ҜёЖИЬТә | CO2УлCaCl2І»·ҙУҰ |
D | ЕЁБтЛб | ХбМЗ | деЛ®НКЙ« | ЕЁБтЛбҫЯУРНСЛ®РФЎўОьЛ®РФ |
A.AB.BC.CD.D
ЎҫМвДҝЎҝПВұнОӘФӘЛШЦЬЖЪұнөДТ»Іҝ·ЦЈ¬ЗлІОХХФӘЛШўЩ~ўвФЪұнЦРөДО»ЦГЈ¬УГ»ҜС§УГУп»ШҙрПВБРОКМвЈә
Че ЦЬЖЪ | ўсA | ўтA | ўуA | ўфA | ўхA | ўцA | ўчA | 0Че |
1 | ўЩ | |||||||
2 | ўЪ | ўЫ | ўЬ | ўЭ | ||||
3 | ўЮ | ўЯ | ўа | ўб | ўв |
ЈЁ1Ј©ФЪХвР©ФӘЛШЦРЈ¬»ҜС§РФЦКЧоІ»»оЖГөДФӘЛШөДФӯЧУҪб№№КҫТвНјОӘ___Ј¬РОіЙөДөҘЦККЗ°лөјМеөДКЗ___ЈЁМоФӘЛШГыіЖЈ©ЎЈ
ЈЁ2Ј©ўЮУлўбРОіЙөД»ҜәПОпөДөзЧУКҪОӘ___Ј¬ўЩУлўЪРОіЙөДЧојтөҘ»ҜәПОпөДҪб№№КҪОӘ___ЎЈ
ЈЁ3
ЈЁ4Ј©ЖшМ¬Зв»ҜОпЧоОИ¶ЁөДКЗ___ЈЁМо»ҜС§КҪЈ©Ј¬ўЪЎўўЫЎўўаөДФӯЧУ°лҫ¶ЧоРЎКЗ__ЈЁМоЛШ·ыәЕЈ©ЎЈ
ЈЁ5Ј©ўЬУлўаРОіЙөД»ҜәПОпөДКфУЪ___ЈЁМоЎ°АлЧУ»ҜәПОпЎұ»тЎ°№ІјЫ»ҜәПОпЎұЈ©Ј¬ёГҫ§МеКфУЪ___ҫ§МеЈЁМоЎ°АлЧУЎұЎўЎ°·ЦЧУЎұЎўЎ°ФӯЧУЎұЈ©ЎЈ
ЎҫМвДҝЎҝАаНЖЛјО¬КЗ»ҜС§ҪвМвЦРіЈУГөДТ»ЦЦЛјО¬·Ҫ·ЁЈ¬ПВБРУР№ШАлЧУ·ҪіМКҪөДАаНЖХэИ·өДКЗ( )
ТСЦӘ | АаНЖ | |
A | Ҫ«FeјУИлCuSO4ИЬТәЦРFe+Cu2+=Cu+Fe2+ | Ҫ«NaјУИлөҪCuSO4ИЬТәЦР2Na+Cu2+=Cu+2Na+ |
B | ПтCa(ClO)2ИЬТәЦРНЁИлЙЩБҝCO2 Ca2++2ClO-+CO2+H2O=CaCO3Ўэ+2HClO | ПтCa(ClO)2ИЬТәЦРНЁИлЙЩБҝSO2 Ca2++2ClO-+SO2+H2O=CaSO3Ўэ+2HClO |
C | УГ¶иРФөзј«өзҪвNaClИЬТә 2Cl-+2H2O | УГ¶иРФөзј«өзҪвMgBr2ИЬТә 2Br-+2H2O |
D | ПЎСОЛбУлNaOHИЬТә·ҙУҰЦБЦРРФ H++OH-=H2O | ПЎHNO3УлBa(OH)2ИЬТә·ҙУҰЦБЦРРФ H++OH-=H2O |
A.AB.BC.CD.D