��Ŀ����
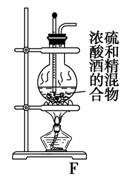
ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
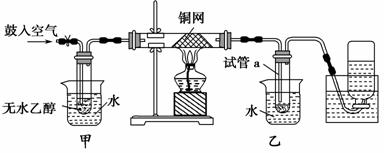
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ��_____________________________________________________________
________________________________________________________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
(2)��������ˮԡ�����ò���ͬ��
��������________���ҵ�������___________________________________��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������________________________________________________________________��
����ƿ���ռ������������Ҫ�ɷ���________________________________��
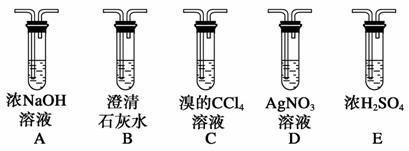
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���________(��д��ĸ)��
a���Ȼ�����Һ b����
c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ��________(��ʵ���������)���ɳ�ȥ��
������(1)���Ҵ��Ĵ�����ʵ���У�Cu����������Ӧ�����У���ɫ��Cu�����ɺ�ɫ��CuO����ɫ��CuO�ֱ��Ҵ���ԭΪ��ɫ��Cu���йصĻ�ѧ����ʽΪ2Cu��O2 2CuO��CuO��CH3CH2OH
2CuO��CuO��CH3CH2OH Cu��CH3CHO��H2O��
Cu��CH3CHO��H2O��
(2)��ˮԡ���ȵ�Ŀ���ǻ��ƽ�ȵ��Ҵ�����������ˮԡ��Ŀ����Ϊ��������ȩ��
(3)���ɵ�CH3CHO��H2O�Լ��ӷ��������Ҵ������Թ�a�������ռ���������ˮ��N2���ռ��ڼ���ƿ�С�
(4)��ʹ��ɫʯ����ֽ�Ժ�ɫ��˵����Һ��Ϊ�������ʣ���CH3COOH��Ҫ��ȥ��ȩ�е����ᣬ�����Ƚ�����NaHCO3��Ӧ����CH3COONa���ټ�����������CH3CHO��
�𰸡�(1)2Cu��O2 2CuO��
2CuO��
CH3CH2OH��CuO CH3CHO��H2O��Cu������
CH3CHO��H2O��Cu������
(2)���ȡ���ȴ��(3)��ȩ���Ҵ���ˮ������
(4)���ᡡc������

 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д� iCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��_______________________________________��
iCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��_______________________________________�� 3SiCl4(g)��2N2(g)��6H2(g)
3SiCl4(g)��2N2(g)��6H2(g) Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)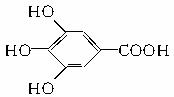
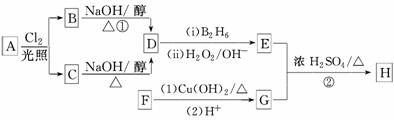
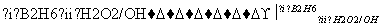 RCH2CH2OH(����B2H6������)
RCH2CH2OH(����B2H6������)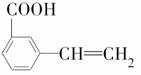 ��
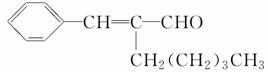
��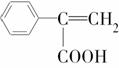 ���������ֵĽṹ��ʽ�ֱ���______________
���������ֵĽṹ��ʽ�ֱ���______________