��Ŀ����
���е������Һ����Na2CO3��NaHCO3��NaAlO2��CH3COONa��NaOH������֪��CO2+3H2O+2AlO2-�T2Al��OH��3��+CO32-����1����������Һ��pH��ͬʱ�������ʵ���Ũ���ɴ�С��˳����______�����ţ���
��2�����������ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��������Һϡ����ͬ����ʱ����pH�仯������______�����ţ���
��3�����������ֵ������Һ�зֱ����AlCl3��Һ���������������______�����ţ�
��4���������١��ڡ��ۡ��������ֵ������Һ��ϣ�������Ӧ�����ӷ���ʽΪ______��
��2�������Ƿ����ƽ������жϣ�������ƽ���PHֵ�仯��
��3��̼�����̼������������ӷ�������˫ˮ�ⷴӦ�������������������̼��
��4��̼�������ܵ���������ӣ������Ӻ�ƫ�����Ʒ�Ӧ������ǿ����ȡ���ᣮ
����⣺��1���١��ڡ��ۡ������Σ����ǼPHֵ��ͬ�����Ũ����С���١��ڡ��ۡ��������ε���������ͬ���������Ӷ�Ӧ����Խ���������ˮ��̶�Խ�����ԣ����̼�̼�������ƫ���ᣬPHֵ��ͬ�������ˮ��̶�Խ�������ʵ���Ũ��ԽС����Ũ�Ȣܣ��ڣ��٣��ۣ�����Һ����ܣ��ڣ��٣��ۣ��ݣ�
�ʴ�Ϊ���ܢڢ٢ۢݣ�
��2���٢ڢۢܶ�����ˮ��ƽ�⣬��ϡ��ʱ���ε��������ˮ����������ӽ��в��䣻����������ǿ���ȫ���룬�����ڵ���ƽ�⣬���Ե�ϡ��ʱ����pH�仯���
�ʴ�Ϊ���ݣ�
��3��̼�����̼������������ӷ�������˫ˮ�ⷴӦ�������������������̼��NaAlO2���Ȼ�������˫ˮ�ⷴӦ������������������CH3COONa���Ȼ�������Ӧ��NaOH���Ȼ�����Ӧ������������������
�ʴ�Ϊ���ۢܢݣ�
��4��̼�������ܵ���������ӣ������Ӻ�ƫ�����Ʒ�Ӧ����̼���ƺ�������������Ӧ���ӷ���ʽΪ��
H2O+HCO3-+AlO2-�TCO32-+Al ��OH��3����
�ʴ�Ϊ��H2O+HCO3-+AlO2-�TCO32-+Al ��OH��3����
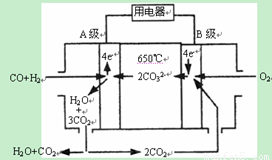
������������Ҫ��������ˮ��ȣ��Ѷ��еȣ�ע�������ˮ����ɵ����⣬ע�����ճ�������˫ˮ�ⷴӦ��

����E��F��G��M��N���ֿ��ܵ�ǿ����ʣ�������ˮ�е�������������ӣ��������Ӳ��ظ�����
|
������ |
H+��Na+��Al3+��Ag+��Ba2+ |
|
������ |
OH-��Cl-��CO32-��NO3-��SO42- |
��֪��
��E��F����Һ�ʼ��ԣ�G��M��N ��Һ�����ԡ�
����N��Һ����εμ�F��Һ�������������������Ӻ���ٵ�����ʧ��
��M��Һ������������Һ��Ӧ���ܲ���������
����˵����ȷ����
A��N��Һ�������F��Һ��Ӧ�����ӷ���ʽ�� Ba2++SO42-=BaSO4��
B��E��Һ��N��Һ��Ϸ�����Ӧ��2Al3++3CO32-+3H2O=2Al(OH)3��+3CO2��
C��M��Һ��F��Һ��ϲ����ij��������ܽ��ڹ�����ˮ��
D����G��Һ��μ��������������ʵ�����Ũ�ȵ�E��Һ�У���Ӧ�����ӷ���ʽΪ
2H++CO32-=CO2��+H2O
����6�֣������������ʣ� ��NaCl���塡��Һ̬SO2���۴����ᡡ�����ᱵ����ͭ �ƾ���C2H5OH�� ���ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ�������������������������������������������������� ��
��2������������ʵ������������������������������������� ��
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ������������������������������ ��
����4�֣�
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð�
������ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���
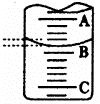
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��
A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��
��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ��
��ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ
������¼���£�
|
ʵ����� |
��һ�� |
�ڶ��� |
������ |
|
����NaOH��Һ���/mL |
26.02 |
25.35 |
25.30 |
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ�
��ԭ����������������������� ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
C����һ�εζ��õ���ƿδ��ϴ
D���ζ�����ʱ�����Ӷ���
��3�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ(���ػ���)��
c������������ ������ ��
����15�֣�
��֪����25ʱH2O H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH
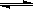 H++ CH3COO����Ka=1.8��10-5
H++ CH3COO����Ka=1.8��10-5
��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH��
�ı�ֵ�� �� �������С�����䡱��
��2��������ˮ������ӷ���ʽΪ���������������� ���������¶�ʱ��C(OH��)����������
���������С�������䡱����
��3��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д�����
�ı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵ
Ϊ������������������ ��a��b�Ĺ�ϵΪ����������������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳�������������������� ������ ��
��5�������������������Һ��Ϻ�pH<7����c��Na+��_______________ c��CH3COO����������ڡ�����С�ڡ����ڡ�����
��6������pH��3��HA��ҺV1mL��pH��11��NaOH{��ҺV2 mL����϶��ã�������˵������ȷ����____________��
A������Ӧ����Һ�����ԣ���c��H+��+c��OH������2��10��7mol��L��1
B����V1=V2����Ӧ����ҺpHһ������7
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2
��7����ij��Һ�к�Mg2+��Cd2+��Zn2+�������ӵ�Ũ�Ⱦ�Ϊ0.01mol��L-1�������м����
������ƺ�����Һ��C(OH-)Ϊ2.2��10-5mol��L-1���������ֽ���������
�������� �����ɳ�����ԭ�������� ��
��KSP��Mg��OH��2��=1.8��10-11��KSP��Zn��OH��2��=1.2��10-17��KSP��Cd��OH��2��=2.5��10-14��
��8��ȡ10mL0.5mol��L-1������Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c��H+��
=������ ����
����E��F��G��M��N���ֿ��ܵ�ǿ����ʣ�������ˮ�е�������������ӣ��������Ӳ��ظ�����
| ������ | H+��Na+��Al3+��Ag+��Ba2+ |
| ������ | OH-��Cl-��CO32-��NO3-��SO42- |
��E��F����Һ�ʼ��ԣ�G��M��N ��Һ�����ԡ�
����N��Һ����εμ�F��Һ�������������������Ӻ���ٵ�����ʧ��
��M��Һ������������Һ��Ӧ���ܲ���������
����˵����ȷ����
- A.N��Һ�������F��Һ��Ӧ�����ӷ���ʽ�� Ba2++SO42-=BaSO4��
- B.E��Һ��N��Һ��Ϸ�����Ӧ��2Al3++3CO32-+3H2O=2Al(OH)3��+3CO2��
- C.M��Һ��F��Һ��ϲ����ij��������ܽ��ڹ�����ˮ��
- D.��G��Һ��μ��������������ʵ�����Ũ�ȵ�E��Һ�У���Ӧ�����ӷ���ʽΪ
2H++CO32-=CO2��+H2O



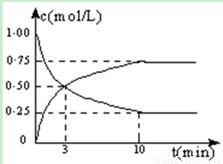
 CH3OH(g)
+ H2O(g) ��H = -49.0 kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)
+ H2O(g) ��H = -49.0 kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��