��Ŀ����
��.������㶨���ܱ������У�����2mol CO2��5mol H2��һ�������·�����Ӧ�� CO2(g) + 3H2(g)  CH3OH(g)
+ H2O(g) ��H = -49.0 kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)
+ H2O(g) ��H = -49.0 kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
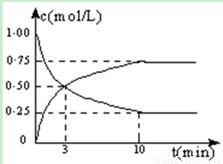
��1���ӷ�Ӧ��ʼ����10min��H2��ת����Ϊ ���� �������£���Ӧ��ƽ�ⳣ��K= �������ijһʱ�̱����¶Ȳ��䣬ֻ�ı�Ũ�ȣ�ʹc(CO2)=1.00mol/L��c(H2)=0.40mol/L��c(CH3OH)=c(H2O)=0.80mol/L����ƽ�� ��ѡ����ţ���
a���������ƶ� b���������ƶ�
c�����ƶ� d����ȷ��ƽ���ƶ�����
��2�����д�ʩ����ʹn(CH3OH)/n(CO2)������� ��ѡ����ţ���
a�������¶� b������He(g)��ʹ��ϵѹǿ����
c����H2O(g)����ϵ�з��� d���ٳ���l mol CH3OH(g)
II������̼����ȼ�ϵ�أ�MCFS����������1889�ꡣ����һ��̼����ȼ�ϵ�أ���һ������Li2CO3��Na2CO3���ۻ����Ϊ����ʣ������¶�Ϊ650�棬�ڴ��¶�������Ϊ��������ú����CO��H2�������Ϊ1:1��ֱ����ȼ�ϣ��乤��ԭ����ͼ��ʾ����ش��������⣺
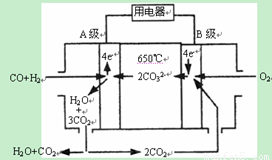
��1��A�缫�ĵ缫��Ӧ����ʽΪ ��
��2�������£���ʯī���缫���Դ˵�Դ���һ������CuSO4 ��Һ�����������������������ͬʱֹͣͨ�磬��������Һ�����Ϊ2L����Һ��pH=1��������ˮ�������H+������������������������ʵ����� ��
��.��1�� 90% �� K= 144 �� a ��2�� c d
II����1�� CO+H2-4e-+2CO32-=3CO2+H2O ��2�� 0.1mol
��������
�����������.��ͼ���ж�CO2��Ũ�Ƚ��ͣ�CH3OH(g)��Ũ�����ߣ� ���������Ϊ2L
CO2(g)
+ 3H2(g)  CH3OH(g)
+ H2O(g)
CH3OH(g)
+ H2O(g)
ʼ�� 2 5 0 0
ת���� 1.5 4.5 1.5 1.5
ƽ���� 0.5 0.5 1.5 1.5
H2��ת����Ϊ4.5��5=0.9
K=1.5/2��1.5/2�£�0.5/2 ��(0.5/2)3��=144
Qc=0.80��0.80�£�1.00��0.403��=10��144����ƽ�������ƶ���
��2��a�����¶�ƽ�������ƶ�����ֵ��С������b�����³���He(g)��ƽ�ⲻ�ƶ�����ֵ���䣬����c��������ƽ�������ƶ�����ֵ�����ȷ��d�ٳ���CH3OH(g)���京����ߣ���ֵ�����ȷ��
II����1��A�缫Ϊ������ע�����Ϊ̼���Σ� CO+H2-4e-+2CO32-=3CO2+H2O ��
��2�����CuSO4 ��Һ�����������������������Ȳ���ͭ���ʣ��������������ӦʽΪ
������4OH- + 4e- = O2��+ 2H2O
������Cu2+ + 2e- = Cu(��)
2H+ + 2e- = H2������
�����������������ȣ����ݵ����غ���ʽΪ4n-2n=0.1��2[����������H+ ��ȥ�������ĵ�H+ ������Һ��ʣ��H+ ] n=0.1mol
���㣺���黯ѧƽ�⼰�绯ѧ�й����⡣

 xC��g�����ﵽƽ���C��ƽ�������е��������Ϊ�գ���ά���¶Ȳ��䣬��1.2molA��0.4molB��0.6molCΪ��ʼ���ʣ�
xC��g�����ﵽƽ���C��ƽ�������е��������Ϊ�գ���ά���¶Ȳ��䣬��1.2molA��0.4molB��0.6molCΪ��ʼ���ʣ�