��Ŀ����
��10�֣������ǹ�ҵ��������Ϊ��Ҫ�IJ�Ʒ֮һ���ڴ���Ӧ������еķ�ӦΪ��
2SO2(g)+O2(g) 2SO3(g) ��H=" �C196.6" kJ��mol-1
2SO3(g) ��H=" �C196.6" kJ��mol-1
��1���÷�Ӧ����������ͨ����400~500�桢 �� ��
��2��SO3�� �����豸���ƣ����� �����Լ����ƣ����ա�
��3���о�SO2 ��NO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��֪�� 2NO(g)+O2(g) 2NO2(g) ��H=" �C113.0" kJ��mol-1
2NO2(g) ��H=" �C113.0" kJ��mol-1
��ӦNO2(g)+SO2(g) SO3(g)+NO(g)�Ħ�H= kJ��mol-1��
SO3(g)+NO(g)�Ħ�H= kJ��mol-1��
һ�������£���NO2��SO2�����ʵ���֮��1:2���ں����ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
a����ϵѹǿ���ֲ���
b�����������ɫ���ֲ���
c��SO3��NO������ȱ��ֲ���
d��ÿ����1 mol SO3��ͬʱ����1 mol NO2
���������Ӧƽ��ʱNO2��SO2�����ʵ���֮��Ϊ1:6����ƽ�ⳣ��K�� ��
��10�֣���1����ѹ ������ÿ��1�֣�
��2�������� 98%���ᣨÿ��1�֣�
��3���C41.8 b 2.67��8/3��ÿ��2�֣�
����

[��ѧ����ѡ��ѧ�뼼��]��15�֣�
�����ǹ�ҵ��������Ϊ��Ҫ�IJ�Ʒ֮һ��
��1����ҵ����������Ҫ��Ϊ_________��________��_________�����Ρ�
��2�������ǵڶ��η�Ӧ���й�ʵ�����ݣ���ѡ������ʵ���������____________��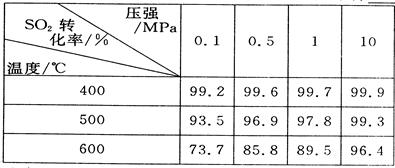
��3����������߷�Ӧ���ʺ�SO2��ת���ʣ����д�ʩ���е��ǣ� ��
| A����װ����ͨ�뵪���ұ���������� |
| B����װ����ͨ�������ұ���������� |
| C�����Ӹ���Ĵ��� |
| D�������¶ȣ���ʱת��SO3 |
��5��������ڶ��������豸��������������Ϊ��SO27%��O211%��N282%������100��������Ļ�������ڷ�Ӧ����96.7�������Ӧ����������N2��SO3�������Ϊ___________����ʱSO2ת����Ϊ__________��
 2SO3(g) ��H= �C196.6 kJ��mol-1
2SO3(g) ��H= �C196.6 kJ��mol-1