��Ŀ����
��A��B��C��D��E ����Ԫ�أ����ǵĺ˵�������������Ҷ�С��20������C��E�ǽ���Ԫ�أ�A��E��ͬһ�壬����ԭ�ӵ����������Ų�Ϊns1��B��DҲ��ͬһ�壬����ԭ��������p�ܼ���������s�ܼ���������������Cԭ��������ϵ���������Dԭ��������ϵ�������һ�롣��ش��������⣺
��1������������Ԫ���У�����S������_____________________________����Ԫ�ط��ţ���
��2����������Ԫ����ɵ�һ�ֻ�������_______________________________��д��ѧʽ����
��3��д��DԪ�ػ�̬ԭ�ӵ����������Ų�ͼ__________________________________��
��4��Ԫ�ص縺��ΪB_______D��Ԫ�ص�һ������ΪC______E���������������������
��1��H K ����1�֣� ��2��KAl(SO4)2?12H2O ��2�֣�
��3��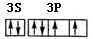 ��2�֣� ��4���� �� ��������1�֣�
��2�֣� ��4���� �� ��������1�֣�
����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ





