��Ŀ����
��8�֣�
 ��֪������Ԫ��T�� Q��R��W��Ԫ�����ڱ��е�λ�����ұ���ʾ������T��������������������������ȣ���ش��������⣺
��֪������Ԫ��T�� Q��R��W��Ԫ�����ڱ��е�λ�����ұ���ʾ������T��������������������������ȣ���ش��������⣺
|
|
Q |
R |
|
|
T |
|
|
W |
��1��T��ԭ�ӽṹʾ��ͼΪ ��
 ��2��Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������W Q���ǿ�ڡ������ڡ�����
��2��Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������W Q���ǿ�ڡ������ڡ�����
��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ ��
 ��4����֪ԭ��������R��1��Ԫ�أ���һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����
��
��4����֪ԭ��������R��1��Ԫ�أ���һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����
��
��5����֪R�ж�����������м���Է���������С����һ�������£�2L�ļ�������0.5L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ���� ��
��1�� ��1�֣� ��2��ǿ�ڣ�1�֣�
��1�֣� ��2��ǿ�ڣ�1�֣�
��3��S��2H2SO4(Ũ) 3SO2����2H2O
3SO2����2H2O
��4��2H2O2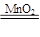 2H2O��O2���������������𰸣���
2H2O��O2���������������𰸣���
��5��NaNO2
����������

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д���8�֣�
��֪������Ԫ��T�� Q��R��W��Ԫ�����ڱ��е�λ�����ұ���ʾ������T��������������������������ȣ���ش��������⣺
|
| Q | R |
|
| T |
|
| W |
��1��T��ԭ�ӽṹʾ��ͼΪ ��
��2��Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������W Q���ǿ�ڡ������ڡ�����
��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ ��
��4����֪ԭ��������R��1��Ԫ�أ���һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ���� ��
��5����֪R�ж�����������м���Է���������С����һ�������£�2L�ļ�������0.5L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ���� ��
��8�֣�
 ��֪������Ԫ��T�� Q��R��W��Ԫ�����ڱ��е�λ�������ʾ������T��������������������������ȣ���ش��������⣺
��֪������Ԫ��T�� Q��R��W��Ԫ�����ڱ��е�λ�������ʾ������T��������������������������ȣ���ش��������⣺
| | Q | R | |
| T | | | W |
 ��1��T��ԭ�ӽṹʾ��ͼΪ ��
��1��T��ԭ�ӽṹʾ��ͼΪ �� ��2��Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������W Q���ǿ�ڡ������ڡ�����
��2��Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������W Q���ǿ�ڡ������ڡ����� ��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ ��
��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ �� ��4����֪ԭ��������R��1��Ԫ�أ���һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ���� ��
��4����֪ԭ��������R��1��Ԫ�أ���һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ���� �� ��5����֪R�ж�����������м���Է���������С����һ�������£�2L�ļ�������0.5L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ���� ��
��5����֪R�ж�����������м���Է���������С����һ�������£�2L�ļ�������0.5L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ���� �� | W | |||||||
| X | Y | Z |
��2��Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ���������ˮ��Һ�����ԣ���ԭ���� ���û�ѧ�����ʾ��������Һ�и�������Ũ���ɴ�С��˳��Ϊ ��
��3����XW4��Z2��KOH��Һ��ɵ�����ȼ�ϵ���У������Ϸ�����Ӧ�ĵ缫��ӦʽΪ ��
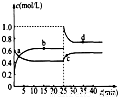
��4����֪��2YZ2��g��?Y2Z4��g������H��0���ں��º��������£���һ����YZ2��Y2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ��ͼ��ʾ��
��a��b��c��d�ĸ����У���ѧ��Ӧ����ƽ��״̬���� �㣮
��25minʱ�������� �������ʵĻ�ѧʽ�� mol��
��a��b��c��d�ĸ���������ʾ�ķ�Ӧ��ϵ�У�������ɫ���dz��˳���� ������ĸ����