��Ŀ����
��֪������Ԫ��W��X��Y��Z����Ԫ�أ�����X��Y��Z����Ԫ�ص�������֮��Ϊ21��W��X��Y��Z��Ԫ�����ڱ��е�λ����ͼ��ʾ��| W | |||||||
| X | Y | Z |
��2��Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ���������ˮ��Һ�����ԣ���ԭ���� ���û�ѧ�����ʾ��������Һ�и�������Ũ���ɴ�С��˳��Ϊ ��
��3����XW4��Z2��KOH��Һ��ɵ�����ȼ�ϵ���У������Ϸ�����Ӧ�ĵ缫��ӦʽΪ ��
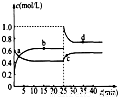
��4����֪��2YZ2��g��?Y2Z4��g������H��0���ں��º��������£���һ����YZ2��Y2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ��ͼ��ʾ��
��a��b��c��d�ĸ����У���ѧ��Ӧ����ƽ��״̬���� �㣮
��25minʱ�������� �������ʵĻ�ѧʽ�� mol��
��a��b��c��d�ĸ���������ʾ�ķ�Ӧ��ϵ�У�������ɫ���dz��˳���� ������ĸ����
���𰸡�������X��Y��Z����Ԫ�ص�������֮��Ϊ21��X��ԭ������Ϊn����Y��Z�ķֱ�Ϊn+1��n+2����n+n+1+n+2=21�����n=6������XΪC��YΪN��ZΪO�����Ԫ�������ڱ��е�λ�ÿ�֪��WΪH��
��1��W��Z�γ�ԭ�Ӹ�����Ϊl��l�Ļ�����ΪH2O2��
��2��Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ��������泥�ˮ�������ԣ�
��3�������ڸ�����ʧȥ���ӣ�
��4������ͼ��֪��Ũ�Ȳ������仯ʱΪƽ��㣻
��c��NO2��˲������ƽ��״̬�����ˣ�1-0.6��×2=0.8mol NO2��
��c��NO2��Խ����ɫԽ�
����⣺X��Y��Z����Ԫ�ص�������֮��Ϊ21��X��ԭ������Ϊn����Y��Z�ķֱ�Ϊn+1��n+2����n+n+1+n+2=21�����n=6������XΪC��YΪN��ZΪO�����Ԫ�������ڱ��е�λ�ÿ�֪��WΪH��
��1��W��Z�γ�ԭ�Ӹ�����Ϊl��l�Ļ�����ΪH2O2�������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ��������泥�ˮ�������ԣ�ˮ�����ӷ�ӦΪNH4++H2O?NH3��H2O+H+��ˮ��̶Ⱥ�С��ˮ�������ԣ�������Ũ�ȴ�С��ϵΪc��NO3-����c��NH4+����c��H+����c��OH-�����ʴ�Ϊ��NH4++H2O?NH3��H2O+H+��c��NO3-����c��NH4+����c��H+����c��OH-����
��3�������ڸ�����ʧȥ���ӣ�������ӦΪCH4-8e-+10OH-=CO32-+7H2O���ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H2O��
��4������ͼ��֪��Ũ�Ȳ������仯ʱΪƽ��㣬��ͼ��b��d����Ϊƽ��㣬�ʴ�Ϊ��bd��
��c��NO2��˲������ƽ��״̬�����ˣ�1-0.6��×2=0.8mol NO2���ʴ�Ϊ��NO2��0.8��
��c��NO2��Խ����ɫԽ�����ɫ���dz��˳����cdba���ʴ�Ϊ��cdba��
���������⿼����ۺϣ��漰λ�á��ṹ�����ʵĹ�ϵ����ѧƽ�⡢����Ũ�ȴ�С�ıȽϵȣ����ط�Ӧԭ���Ŀ��飬ע�ظ߿��������ѵ������Ŀ�Ѷ��еȣ�
��1��W��Z�γ�ԭ�Ӹ�����Ϊl��l�Ļ�����ΪH2O2��
��2��Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ��������泥�ˮ�������ԣ�
��3�������ڸ�����ʧȥ���ӣ�
��4������ͼ��֪��Ũ�Ȳ������仯ʱΪƽ��㣻
��c��NO2��˲������ƽ��״̬�����ˣ�1-0.6��×2=0.8mol NO2��
��c��NO2��Խ����ɫԽ�
����⣺X��Y��Z����Ԫ�ص�������֮��Ϊ21��X��ԭ������Ϊn����Y��Z�ķֱ�Ϊn+1��n+2����n+n+1+n+2=21�����n=6������XΪC��YΪN��ZΪO�����Ԫ�������ڱ��е�λ�ÿ�֪��WΪH��
��1��W��Z�γ�ԭ�Ӹ�����Ϊl��l�Ļ�����ΪH2O2�������ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ��������泥�ˮ�������ԣ�ˮ�����ӷ�ӦΪNH4++H2O?NH3��H2O+H+��ˮ��̶Ⱥ�С��ˮ�������ԣ�������Ũ�ȴ�С��ϵΪc��NO3-����c��NH4+����c��H+����c��OH-�����ʴ�Ϊ��NH4++H2O?NH3��H2O+H+��c��NO3-����c��NH4+����c��H+����c��OH-����
��3�������ڸ�����ʧȥ���ӣ�������ӦΪCH4-8e-+10OH-=CO32-+7H2O���ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H2O��
��4������ͼ��֪��Ũ�Ȳ������仯ʱΪƽ��㣬��ͼ��b��d����Ϊƽ��㣬�ʴ�Ϊ��bd��
��c��NO2��˲������ƽ��״̬�����ˣ�1-0.6��×2=0.8mol NO2���ʴ�Ϊ��NO2��0.8��
��c��NO2��Խ����ɫԽ�����ɫ���dz��˳����cdba���ʴ�Ϊ��cdba��
���������⿼����ۺϣ��漰λ�á��ṹ�����ʵĹ�ϵ����ѧƽ�⡢����Ũ�ȴ�С�ıȽϵȣ����ط�Ӧԭ���Ŀ��飬ע�ظ߿��������ѵ������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
�����Ŀ
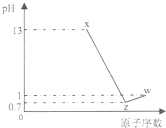 ��֪������Ԫ��A��B��C��D����������Ӧˮ����ֱ�ΪX��Y��Z��W��A�Ƕ�������ԭ�Ӱ뾶����Ԫ�أ�������X��Z��W������Y��Ӧ��A��C��D��ԭ��������0.1 mol/LX��Z��W��Һ��pH��ͼ��ʾ������˵����ȷ���ǣ�������
��֪������Ԫ��A��B��C��D����������Ӧˮ����ֱ�ΪX��Y��Z��W��A�Ƕ�������ԭ�Ӱ뾶����Ԫ�أ�������X��Z��W������Y��Ӧ��A��C��D��ԭ��������0.1 mol/LX��Z��W��Һ��pH��ͼ��ʾ������˵����ȷ���ǣ�������| A��B�����Ӱ뾶����A�����Ӱ뾶 | B��C�⻯���ȶ��Դ���D�⻯���ȶ��� | C��X��W�����ʺ��еĻ�ѧ��������ͬ | D��Bԭ�ӵĵ��Ӳ������������������ |

