��Ŀ����
ͼʾ����ͼ���dz��õĿ�ѧ�о�������
��1����ѧ��ͨ��X�����Ʋ���мȺ�����λ���ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�������λ����������������߱�ʾ��
��д����̬Cuԭ�ӵĺ�������Ų�ʽ____________________��
��д������������ˮ��ͭ���ӵĽṹ��ʽ�����뽫��λ����ʾ������________��
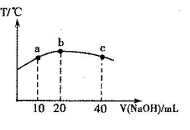
��2����ͼ���о�����Ԫ���⻯��ķе�仯���ɵ�ͼ������c���Ա������________��Ԫ���⻯��ķе�仯���ɡ���λͬѧ��ij����Ԫ���⻯��ķе�ı仯���ƻ������������ߡ�������a������b������Ϊ��ȷ����________���a����b������������______________________________________________________________��
��3����������Ԫ�ص���̬�����Ի�̬ԭ��ʧȥ�����һ������ת��Ϊ��̬��̬������������������������һ�����ܣ���ΪE������ͼ��ʾ��
��ͬ�����ڣ�����ԭ������������Eֵ�仯����������________��
�ڸ���ͼ���ṩ����Ϣ�����ƶ�E��________E�������������������������ͬ����
�۸��ݵ�һ�����ܵĺ����Ԫ�������ɣ����ƶ�Eþ________E����
��1����1s22s22p63s23p63d104s1��[Ar]3d104s1 ��
��
��2����A��b��A���ʾ���⻯����ˮ����е����������ˮ����֮���������������ǿ��Զ���ڷ��»�������������Ԫ���������⻯��ķе㲻�����ˮ
��3�������ڣ����ۣ�
����

X��Y��Z��W��R��Ԫ�����ڱ�ǰ�������еij���Ԫ��,�������Ϣ���±�:
| Ԫ�� | �����Ϣ |
| X | ��ɵ����ʵĻ���Ԫ��,����������ϼ���������ϼ۵Ĵ�����Ϊ2 |
| Y | �ؿ��к�����ߵ�Ԫ�� |
| Z | ����������Ϊ23,������Ϊ11�ĺ��� |
| W | �����д���ʹ����Ͻ���Ʒ,��ҵ�Ͽ��õ������������ķ����Ʊ��䵥�� |
| R | �ж��ֻ��ϼ�,���ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ,����ɺ��ɫ |
(1)W��Ԫ�����ڱ��е�λ��Ϊ������;X��Y��Z��W����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳������������������(��Ԫ�ط��ű�ʾ)��
(2)X������Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���A��B,A�ĽṹʽΪ������;B�ĵ���ʽΪ����������������ZY�д��ڵĻ�ѧ������Ϊ����������
(3)��(As)������������Ԫ��,��Xͬһ����,Asԭ�ӱ�Xԭ�Ӷ��������Ӳ�,�����ԭ������Ϊ������,������������Ӧ��ˮ����Ļ�ѧʽΪ���������������������������Ԫ�ص���̬�⻯����ȶ��ԴӴ�С��˳������������������������������(�û�ѧʽ��ʾ)��
(4)��RCl3��Һ��ʴͭ��·������ӷ���ʽΪ����������������������������������Һ��R3+���õ��Լ�����������,���Թ۲쵽����������������������������
(5)Z
 W�Ͻ�(Z17W12)��һ��DZ�ڵ��������,��Z��W������һ���������������ɡ��úϽ���һ����������ȫ����ķ�Ӧ����ʽΪ:Z17W12+17H2
W�Ͻ�(Z17W12)��һ��DZ�ڵ��������,��Z��W������һ���������������ɡ��úϽ���һ����������ȫ����ķ�Ӧ����ʽΪ:Z17W12+17H2 17ZH2+12W,�õ��Ļ����Q(17ZH2+12W)��6.0 mol��L-1 HCl��Һ������ȫ�ͷų�H2��1 mol Z17W12��ȫ�����õ��Ļ����Q������������ȫ��Ӧ,�ͷų�H2�����ʵ���Ϊ��������
17ZH2+12W,�õ��Ļ����Q(17ZH2+12W)��6.0 mol��L-1 HCl��Һ������ȫ�ͷų�H2��1 mol Z17W12��ȫ�����õ��Ļ����Q������������ȫ��Ӧ,�ͷų�H2�����ʵ���Ϊ�������� 
 ��
��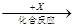 ���������м��ǵ��ʣ��ҡ���Ϊ�����x�Ǿ��������Ե���ɫ���嵥�ʣ���Ļ�ѧ��ɲ�������________(ѡ����ţ���ͬ)��
���������м��ǵ��ʣ��ҡ���Ϊ�����x�Ǿ��������Ե���ɫ���嵥�ʣ���Ļ�ѧ��ɲ�������________(ѡ����ţ���ͬ)��