题目内容
(20分)Ⅰ、醉驾对人们的安全危害很大,利用下列原理可以检查司机是否酒后开车。
2K2Cr2O7(橙色)+3C2H5OH+H2SO4 →Cr2(SO4)3(绿色)+K2SO4+CH3COOH+H2O
①配平化学方程式后,H2O前面的系数为 ;
②怎样判断司机是酒后开车: 。
③写出用粮食酿酒的化学方程式: ; 。
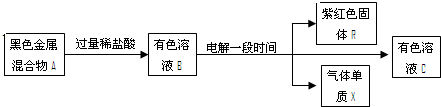
Ⅱ、下列框图中,A由两种黑色金属氧化物等物质的量混合而成,B中含有四种阳离子。据此回答下列问题:
(1)A的组成是 (填化学式)。
(2)相同条件下,溶液B中所有阳离子的氧化性由强到弱的顺序依次是 。
(3)A中某组分可由单质与水反应制得,化学方程式为: 。
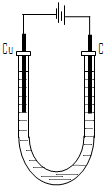
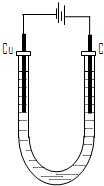
(4)电解所用装置如图所示。
①电解开始阶段,阳极上的电极反应是 ,阴极上的电极反应是 。
②电解至阴极刚开始有固体R析出时,该溶液中金属离子浓度由大到小的顺序是 。
(20分,每空2分)
Ⅰ、①11;
② 将司机呼出的气体通入橙色溶液中,观察溶液颜色是否向绿色转变。
③ (C6H10O5)n + nH2O nC6H12O6 ;C6H12O6
2C2H5OH+2CO2↑
Ⅱ、(1) Fe3O4; CuO 。(2) Fe3+ ;Cu2+;H+ ; Fe2+(全对给分)。
(3) 3Fe+4H2O(g) Fe3O4+ 4H2。
(4)①阳极 2Cl-―2e- = Cl2↑,阴极 2 Fe3++2e- = 2 Fe2+(电子转移不等不给分)。
②Fe2+,Cu2+或者Fe2+,Cu2+,Fe3+。
解析:

 备战中考寒假系列答案
备战中考寒假系列答案