��Ŀ����
�����ݶ����ǵİ�ȫΣ���ܴ���������ԭ�����Լ��˾���Ƿ�ƺ���
2K2Cr2O7����ɫ��+3C2H5OH+H2SO4��Cr2��SO4��3����ɫ��+K2SO4+CH3COOH+H2O
����ƽ��ѧ����ʽ��H2Oǰ���ϵ��Ϊ
�������ж�˾���Ǿƺ���
��д������ʳ��ƵĻ�ѧ����ʽ��
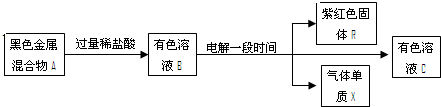
�����п�ͼ�У�A�����ֺ�ɫ��������������ʵ�����϶��ɣ�B�к������������ӣ��ݴ˻ش��������⣺

��1��A�������
��2����ͬ�����£���ҺB�����������ӵ���������ǿ������˳��������
��3��A��ij��ֿ��ɵ�����ˮ��Ӧ�Ƶã���ѧ����ʽΪ��
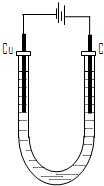
��4���������װ����ͼ��ʾ��
�ٵ�ʼ�Σ������ϵĵ缫��Ӧ��
�����ϵĵ缫��Ӧ��
�ڵ���������տ�ʼ�й���R����ʱ������Һ�н�������Ũ���ɴ�С
��˳����
2K2Cr2O7����ɫ��+3C2H5OH+H2SO4��Cr2��SO4��3����ɫ��+K2SO4+CH3COOH+H2O
����ƽ��ѧ����ʽ��H2Oǰ���ϵ��Ϊ
11
11
���������ж�˾���Ǿƺ���
��˾������������ͨ���ɫ��Һ�У��۲���Һ��ɫ�Ƿ�����ɫת��
��˾������������ͨ���ɫ��Һ�У��۲���Һ��ɫ�Ƿ�����ɫת��
����д������ʳ��ƵĻ�ѧ����ʽ��
��C6H10O5��n+nH2O
nC6H12O6��C6H12O6
2C2H5OH+2CO2��
| ||
| ||
| ��ĸ�� |
��C6H10O5��n+nH2O
nC6H12O6��C6H12O6
2C2H5OH+2CO2��
��
| ||
| ||
| ��ĸ�� |
�����п�ͼ�У�A�����ֺ�ɫ��������������ʵ�����϶��ɣ�B�к������������ӣ��ݴ˻ش��������⣺

��1��A�������
Fe3O4��CuO
Fe3O4��CuO
���ѧʽ������2����ͬ�����£���ҺB�����������ӵ���������ǿ������˳��������
Fe3+��Cu2+��H+��Fe2+��ȫ�Ը��֣�
Fe3+��Cu2+��H+��Fe2+��ȫ�Ը��֣�
����3��A��ij��ֿ��ɵ�����ˮ��Ӧ�Ƶã���ѧ����ʽΪ��
3Fe+4H2O��g��
Fe3O4+4H2
| ||
3Fe+4H2O��g��
Fe3O4+4H2
��
| ||
��4���������װ����ͼ��ʾ��

�ٵ�ʼ�Σ������ϵĵ缫��Ӧ��
2Cl--2e-=Cl2��
2Cl--2e-=Cl2��
�������ϵĵ缫��Ӧ��
2 Fe3++2e-=2 Fe2+
2 Fe3++2e-=2 Fe2+
���ڵ���������տ�ʼ�й���R����ʱ������Һ�н�������Ũ���ɴ�С
��˳����
Fe2+��Cu2+
Fe2+��Cu2+
���������ٸ���ԭ���غ�����ƽ��ѧ����ʽ��
��K2Cr2O7����ɫ�����Ҵ���ɫ�����Լ����Ҵ��Ĵ������
����ʳ��ƵĹ����ǵ�����������ת��Ϊ�����ǣ������Ǿ�����������ת��Ϊ�ƾ���
��1�������ĺ�ɫ������������������������ͭ�ȣ�
��2�����ʵĻ�ԭ��Խǿ�����ӵ�������Խ����������۵�Ԫ�ؾ��н�ǿ�������ԣ�
��3�����������Ժ�ˮ������Ӧ����������������������
��4���ٵ�������������������Ӧ������������ԭ��Ӧ��
���������ϣ�������ǿ�������ȷŵ磮
��K2Cr2O7����ɫ�����Ҵ���ɫ�����Լ����Ҵ��Ĵ������
����ʳ��ƵĹ����ǵ�����������ת��Ϊ�����ǣ������Ǿ�����������ת��Ϊ�ƾ���
��1�������ĺ�ɫ������������������������ͭ�ȣ�
��2�����ʵĻ�ԭ��Խǿ�����ӵ�������Խ����������۵�Ԫ�ؾ��н�ǿ�������ԣ�
��3�����������Ժ�ˮ������Ӧ����������������������
��4���ٵ�������������������Ӧ������������ԭ��Ӧ��
���������ϣ�������ǿ�������ȷŵ磮
����⣺�ٸ��ݼ�ԭ���غ㣬��Ӧ���м�ԭ����ĿΪ4����������K2SO4ϵ��ӦΪ2��3C2H5OH��̼ԭ����ĿΪ6������CH3COOH��ĿӦΪ3��2K2Cr2O7�к���ԭ����Ϊ4������������Cr2��SO4��3��ϵ��Ϊ2������ԭ���غ㣬����ǰ��ϵ��ӦΪ8����Ӧ���й�����34����ԭ�ӣ�������3CH3COOH����ԭ����ĿΪ12������22����ԭ�ӣ�����ˮǰϵ��Ϊ11���ʴ�Ϊ��11��
��K2Cr2O7Ϊ��ɫ�����Ҵ���Ϊ��ɫ�����Խ�˾������������ͨ���ɫ��Һ�У��۲���Һ��ɫ�Ƿ�����ɫת����֤�����������Ƿ����Ҵ����ʴ�Ϊ����˾������������ͨ���ɫ��Һ�У��۲���Һ��ɫ�Ƿ�����ɫת�䣻
����ʳ��ƵĹ����ǵ�����ת��Ϊ�����ǣ������Ǿ�����������ת��Ϊ�ƾ����ʴ�Ϊ����C6H10O5��n+nH2O
nC6H12O6��C6H12O6
2C2H5OH+2CO2����
��1�������ĺ�ɫ������������������������ͭ�ȣ�������ҺB�ĵ������֪A�������Fe3O4��CuO���ʴ�Ϊ��Fe3O4��CuO��
��2�����ʵĻ�ԭ��Խǿ�����ӵ�������Խ�������������ԣ�Cu2+��H+��Fe2+��������������ۣ����е������Ա�ͭ����ǿ���ʴ�Ϊ��Fe3+��Cu2+��H+��Fe2+��
��3�����������Ժ�ˮ������Ӧ������������������������Ӧ����ʽΪ��3Fe+4H2O��g��
Fe3O4+4H2���ʴ�Ϊ��3Fe+4H2O��g��
Fe3O4+4H2��
��4���ٵ���������Ϊ���Ե缫ʱ�������������ӷ���ʧ���ӵ�������Ӧ�������������ӷ����õ��ӵĻ�ԭ��Ӧ���ʴ�Ϊ��2Cl--2e-=Cl2���� 2 Fe3++2e-=2 Fe2+��
���������ϣ�������ǿ�������ȷŵ磬���ȷ����ĵ缫��ӦΪ��Fe3++e-=Fe2+��Ȼ��ΪCu2++2e-=Cu�������տ�ʼ�й���ͭ����ʱ����������ȫ��װ��Ϊ������������Fe2+Ũ����������Cu2+���ʴ�Ϊ��Fe2+��Cu2+��
��K2Cr2O7Ϊ��ɫ�����Ҵ���Ϊ��ɫ�����Խ�˾������������ͨ���ɫ��Һ�У��۲���Һ��ɫ�Ƿ�����ɫת����֤�����������Ƿ����Ҵ����ʴ�Ϊ����˾������������ͨ���ɫ��Һ�У��۲���Һ��ɫ�Ƿ�����ɫת�䣻
����ʳ��ƵĹ����ǵ�����ת��Ϊ�����ǣ������Ǿ�����������ת��Ϊ�ƾ����ʴ�Ϊ����C6H10O5��n+nH2O
| ||
| ||
| ��ĸ�� |
��1�������ĺ�ɫ������������������������ͭ�ȣ�������ҺB�ĵ������֪A�������Fe3O4��CuO���ʴ�Ϊ��Fe3O4��CuO��
��2�����ʵĻ�ԭ��Խǿ�����ӵ�������Խ�������������ԣ�Cu2+��H+��Fe2+��������������ۣ����е������Ա�ͭ����ǿ���ʴ�Ϊ��Fe3+��Cu2+��H+��Fe2+��
��3�����������Ժ�ˮ������Ӧ������������������������Ӧ����ʽΪ��3Fe+4H2O��g��
| ||
| ||
��4���ٵ���������Ϊ���Ե缫ʱ�������������ӷ���ʧ���ӵ�������Ӧ�������������ӷ����õ��ӵĻ�ԭ��Ӧ���ʴ�Ϊ��2Cl--2e-=Cl2���� 2 Fe3++2e-=2 Fe2+��
���������ϣ�������ǿ�������ȷŵ磬���ȷ����ĵ缫��ӦΪ��Fe3++e-=Fe2+��Ȼ��ΪCu2++2e-=Cu�������տ�ʼ�й���ͭ����ʱ����������ȫ��װ��Ϊ������������Fe2+Ũ����������Cu2+���ʴ�Ϊ��Fe2+��Cu2+��
������������һ���й��������ʵ��ƶ��⣬��Ŀ�ѶȽϴ�˼ά�ռ�㣬����ѧ����֪ʶ�������̶ȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ