��Ŀ����
Ϊ�ⶨþ���Ͻ𣨲�������Ԫ�أ������������������ס��ҡ�������ѧϰС��������������ֲ�ͬ��ʵ�鷽������̽������ش��������⣺
��һ�����飺ʵ�鷽����þ���Ͻ�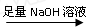 �ⶨʣ���������
�ⶨʣ���������
ʵ�鲽�裺
�ٳ�����������ƽ����һ������þ���Ͻ��ĩ
���ܽ⣺������ҩƷ�����ձ��У����������NaOH��Һ�����Ͻ��裬��ַ�Ӧ��������Ӧ�����ӷ���ʽΪ
�۹��ˣ�
��ϴ�ӣ���δ�Թ������ù������ϴ�ӣ������������������ ���ƫ�ߡ�����ƫ�͡����䡱��
�ݸ������ʣ�����
 ���������飺ʵ�鷽����þ���Ͻ�
���������飺ʵ�鷽����þ���Ͻ� �ⶨ������������
�ⶨ������������
ʵ��װ��������ͼ
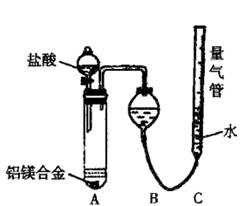
��1��ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ��װ�м�ʯ�ҵĸ���װ�á��������� �����Ҫ������Ҫ����
��2��Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע��������ǣ�Ҫ��д�����㣩 ��
���������飺ʵ�鷽����12 gþ���Ͻ�
����l�������ˡ�ϴ�ӡ�����ͳ����������յõ���������1��45g����úϽ���������������Ϊ ��
��һ��2Al +2OH�� + 6H2O = 2[Al��OH��4] �� + 3H2�� ��2�� ��
����2Al +2OH�� + 2H2O = 2Al O2�� + 3H2����
ƫ�ͣ�1�֣�
����������Ҫ��1�֣�
����װ�õ����������ã�����������C�ĸ߶�ʹC��Һ����B��Һ����ƽ�ٶ�������ȴ�������ٶ���������ʱ����Ӧ�밼Һ����ʹ���ƽ�Ⱥ����𰸾���
��Ҫ��д�����㣬ÿ��1�֣���2�֣�
������95% ��3�֣�
��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�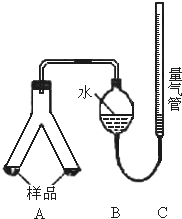
 ���������飺ʵ�鷽����þ���Ͻ�
���������飺ʵ�鷽����þ���Ͻ� ��һ�����飺ʵ�鷽����þ���Ͻ�
��һ�����飺ʵ�鷽����þ���Ͻ�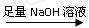 �ⶨʣ���������
�ⶨʣ��������� ����ƽ����һ������þ���Ͻ��ĩ
����ƽ����һ������þ���Ͻ��ĩ ���������飺ʵ�鷽����þ���Ͻ�
���������飺ʵ�鷽����þ���Ͻ� �ⶨ������������
�ⶨ������������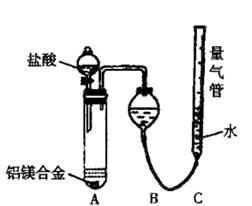

 �����յõ���������1��45g����úϽ���������������Ϊ ��
�����յõ���������1��45g����úϽ���������������Ϊ ��
