��Ŀ����
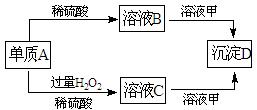
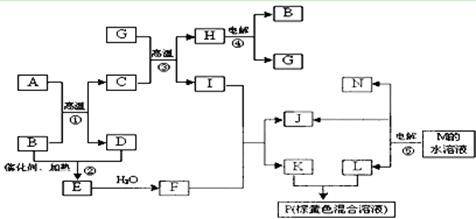
��10�֣�A��B��C��D�ͼ�������ת����ϵ����֪���ʼ��Ƕ�����Ԫ����ɵ��Σ�����ij ������Һ����Ч�ɷ֣�����D�������ᡣ
������Һ����Ч�ɷ֣�����D�������ᡣ
��ش��������⣺
��1�����A��Ԫ�������ڱ���λ�ڵ� ���ڵ� �塣
��2������C�������ӣ�������H+���IJ����������� ��
��3��Aת��ΪBʱ��ų���ɫ����E����298Kʱ1mol A��ȫ��Ӧ�ų�����QkJ����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��4����Aת��ΪCʱ������ų���д���÷�Ӧ�Ļ�ѧ����ʽ��
��
��5��д����Һ C����Һ��Ӧ�����ӷ���ʽ�� ��
C����Һ��Ӧ�����ӷ���ʽ�� ��
 ������Һ����Ч�ɷ֣�����D�������ᡣ
������Һ����Ч�ɷ֣�����D�������ᡣ
��ش��������⣺
��1�����A��Ԫ�������ڱ���λ�ڵ� ���ڵ� �塣
��2������C�������ӣ�������H+���IJ����������� ��
��3��Aת��ΪBʱ��ų���ɫ����E����298Kʱ1mol A��ȫ��Ӧ�ų�����QkJ����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��4����Aת��ΪCʱ������ų���д���÷�Ӧ�Ļ�ѧ����ʽ��
��
��5��д����Һ
 C����Һ��Ӧ�����ӷ���ʽ�� ��
C����Һ��Ӧ�����ӷ���ʽ�� ����10�֣� (1)�� VIII
(2)������C��Һ�е���KSCN��Һ����Һ�Ժ�ɫ��˵��C����Fe3+���ӣ����������𰸾��ɣ�
(3)Fe(s) + 2H+(aq)�� Fe2+(aq)+ H2(g)����H=" -QkJ/mol"
(4)2 Fe +3H2O2 + 3H2SO4�� Fe2(SO4)3 + 6H2O
(5)Fe3+ + 3ClO��+ 3H2O �� Fe(OH)3��+ 3HClO
(2)������C��Һ�е���KSCN��Һ����Һ�Ժ�ɫ��˵��C����Fe3+���ӣ����������𰸾��ɣ�
(3)Fe(s) + 2H+(aq)�� Fe2+(aq)+ H2(g)����H=" -QkJ/mol"
(4)2 Fe +3H2O2 + 3H2SO4�� Fe2(SO4)3 + 6H2O
(5)Fe3+ + 3ClO��+ 3H2O �� Fe(OH)3��+ 3HClO
��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ

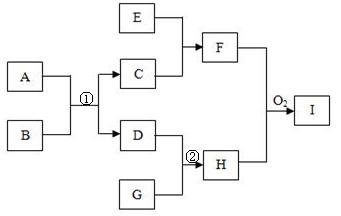
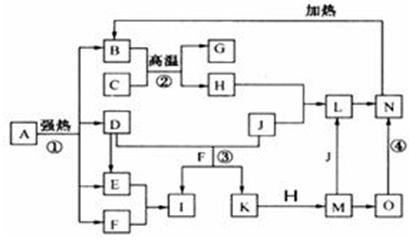
 ��D��F��N��OΪ��ɫ���壬E���³�ѹ��Ϊ��ɫ��ζ��Һ�壬N��H��LΪ���г����ĵ��ʣ�IΪ��������ǿ�ᣬM����ɫ��ӦΪ��ɫ����Ӧ�ٳ���������F�ļ��顣
��D��F��N��OΪ��ɫ���壬E���³�ѹ��Ϊ��ɫ��ζ��Һ�壬N��H��LΪ���г����ĵ��ʣ�IΪ��������ǿ�ᣬM����ɫ��ӦΪ��ɫ����Ӧ�ٳ���������F�ļ��顣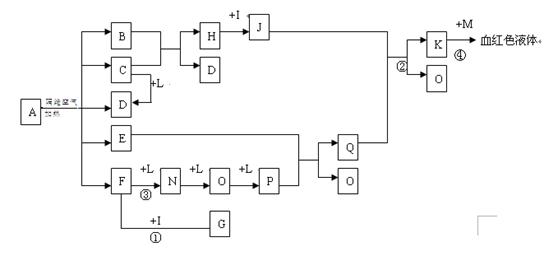
 ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ����ͼ��ʾ������A
��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ����ͼ��ʾ������A ��DΪ�������ʣ���Ӧ������
��DΪ�������ʣ���Ӧ������ ���ɵ�ˮ���������ֲ�������ȥ��
���ɵ�ˮ���������ֲ�������ȥ��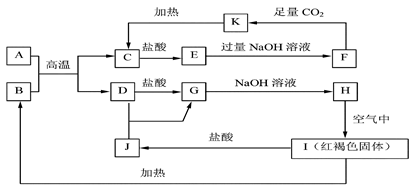
 ��
��
 ��
��
 ______��
______�� �� ��
�� ��
 ��
��