��Ŀ����
A��B��C��D��E������������Ԫ�ؾ���������ͬ������ɫ��Ӧ��Ϊ��ɫ��B��A��Ը��ȶ���C��D�ǹ�ҵ����Ҫ �Ļ���ԭ�ϣ�Ҳ��ʵ���ҳ��õ�ҩƷ��C��D��һ���������¿��ת����F��A��B��C��D��һ���������¾��ɷ�����Ӧ�����ǵIJ���ת����ϵ����ͼ�����ַ�Ӧ���������ʡ�ԣ���
�Ļ���ԭ�ϣ�Ҳ��ʵ���ҳ��õ�ҩƷ��C��D��һ���������¿��ת����F��A��B��C��D��һ���������¾��ɷ�����Ӧ�����ǵIJ���ת����ϵ����ͼ�����ַ�Ӧ���������ʡ�ԣ���

��1��B�ĵ���ʽ ��C������Ϊ ��
��2���ڢ٢ڢۢܢ��У�����������ԭ��Ӧ���� ��
��3��д��E��Һ��C��Һ��Ӧ�����ӷ���ʽ�� ��
��4��д��Dת��ΪC�Ļ�ѧ����ʽ�� ��
 �Ļ���ԭ�ϣ�Ҳ��ʵ���ҳ��õ�ҩƷ��C��D��һ���������¿��ת����F��A��B��C��D��һ���������¾��ɷ�����Ӧ�����ǵIJ���ת����ϵ����ͼ�����ַ�Ӧ���������ʡ�ԣ���
�Ļ���ԭ�ϣ�Ҳ��ʵ���ҳ��õ�ҩƷ��C��D��һ���������¿��ת����F��A��B��C��D��һ���������¾��ɷ�����Ӧ�����ǵIJ���ת����ϵ����ͼ�����ַ�Ӧ���������ʡ�ԣ���
��1��B�ĵ���ʽ ��C������Ϊ ��
��2���ڢ٢ڢۢܢ��У�����������ԭ��Ӧ���� ��
��3��д��E��Һ��C��Һ��Ӧ�����ӷ���ʽ�� ��
��4��д��Dת��ΪC�Ļ�ѧ����ʽ�� ��
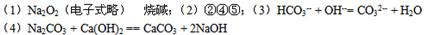
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 ��+��+ˮ��
��+��+ˮ�� ��
�� ��
�� MnO4- +
MnO4- +  2SO3(g) ��H< 0��Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K= �������ʽ������Ӧ��ƽ��ʱ�����ı�����һ������x�������ͼ�����ߵ��� ������ţ���
2SO3(g) ��H< 0��Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K= �������ʽ������Ӧ��ƽ��ʱ�����ı�����һ������x�������ͼ�����ߵ��� ������ţ���




 ������Ԫ��A��B��C��D��Eԭ��������������AԪ�ص��ʳ��³�ѹ������������壬BԪ�����γɻ�����������࣬C������������Ӧˮ�����������̬�⻯�����ܹ������γ��α���DԪ�ص����Ӱ뾶��ͬ����Ԫ���γɵļ���������С�ġ�
������Ԫ��A��B��C��D��Eԭ��������������AԪ�ص��ʳ��³�ѹ������������壬BԪ�����γɻ�����������࣬C������������Ӧˮ�����������̬�⻯�����ܹ������γ��α���DԪ�ص����Ӱ뾶��ͬ����Ԫ���γɵļ���������С�ġ�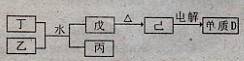
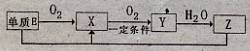
 ������R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ�� ��
������R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ�� ��