��Ŀ����
�״���һ�����ʵ�Һ��ȼ�ϣ�CO��CO2�������ںϳɼ״���
Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������·�����Ӧ��
CO2(g) +3H2(g) ��CH3OH(g)+H2O(g) ��H1
��1����֪��2CO(g) +O2(g) ��2CO2(g) ��H2
2H2(g)+O2(g) ��2H2O(g) ��H3
��CO(g) + 2H2(g) CH3OH(g)�ġ�H�� ��
CH3OH(g)�ġ�H�� ��
��2����CO�ϳɼ״�ʱ��CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ����ͼ��ʾ��
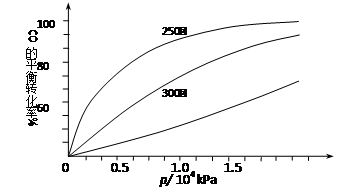
�÷�Ӧ��H 0���>���� <������ʵ����������������250�桢1.3��104kPa���ң�ѡ���ѹǿ�������� ��
��3��
һ���¶��£���2L�ܱ������м���1mol CH3OH (g)��������Ӧ��CH3OH(g) CO(g) + 2H2(g)��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0~2 min�ڵ�ƽ����Ӧ����v(CH3OH)= ��
CO(g) + 2H2(g)��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0~2 min�ڵ�ƽ����Ӧ����v(CH3OH)= ��
���¶��£�CO(g) + 2H2(g) CH3OH(g)��ƽ�ⳣ��K= ��
CH3OH(g)��ƽ�ⳣ��K= ��
��ͬ�¶��£�����ʼ����CH3OH(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a��ƽ�ⳣ�� b��CH3OH��ƽ��Ũ�� c���ﵽƽ���ʱ�� d��ƽ��ʱ������ܶ�
��4����CH3OHΪȼ�ϣ���KOH��Һ���������Һ�����Ƴ�CH3OHȼ�ϵ�ء�
�ٳ���CH3OH�ĵ缫Ϊ ����
�ڸ�����Ӧ�ĵ缫��ӦʽΪ ��
Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������·�����Ӧ��
CO2(g) +3H2(g) ��CH3OH(g)+H2O(g) ��H1
��1����֪��2CO(g) +O2(g) ��2CO2(g) ��H2
2H2(g)+O2(g) ��2H2O(g) ��H3
��CO(g) + 2H2(g)
 CH3OH(g)�ġ�H�� ��
CH3OH(g)�ġ�H�� ����2����CO�ϳɼ״�ʱ��CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ����ͼ��ʾ��

�÷�Ӧ��H 0���>���� <������ʵ����������������250�桢1.3��104kPa���ң�ѡ���ѹǿ�������� ��
��3��

һ���¶��£���2L�ܱ������м���1mol CH3OH (g)��������Ӧ��CH3OH(g)
 CO(g) + 2H2(g)��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0~2 min�ڵ�ƽ����Ӧ����v(CH3OH)= ��
CO(g) + 2H2(g)��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0~2 min�ڵ�ƽ����Ӧ����v(CH3OH)= �����¶��£�CO(g) + 2H2(g)
 CH3OH(g)��ƽ�ⳣ��K= ��
CH3OH(g)��ƽ�ⳣ��K= ����ͬ�¶��£�����ʼ����CH3OH(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a��ƽ�ⳣ�� b��CH3OH��ƽ��Ũ�� c���ﵽƽ���ʱ�� d��ƽ��ʱ������ܶ�
��4����CH3OHΪȼ�ϣ���KOH��Һ���������Һ�����Ƴ�CH3OHȼ�ϵ�ء�
�ٳ���CH3OH�ĵ缫Ϊ ����
�ڸ�����Ӧ�ĵ缫��ӦʽΪ ��
��1����H1+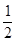 ��H2 ��
��H2 ��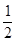 ��H3 ����2�֣�
��H3 ����2�֣�
��2��<����1�֣� ��1.3��104kPa�£�CO��ת�����Ѿ��ܸߣ��������ѹǿCO��ת������߲��������ɱ����ӣ��ò���ʧ����3�֣�
��3��0.125mol��L��1�� min��1����2�֣� 4 L2��mol��2����2�֣� d����1�֣�
��4���ٸ�����1�֣� ��CH3OH �� 6e��+8OH��= CO32��+6H2O����2�֣�
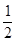 ��H2 ��
��H2 ��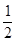 ��H3 ����2�֣�
��H3 ����2�֣���2��<����1�֣� ��1.3��104kPa�£�CO��ת�����Ѿ��ܸߣ��������ѹǿCO��ת������߲��������ɱ����ӣ��ò���ʧ����3�֣�
��3��0.125mol��L��1�� min��1����2�֣� 4 L2��mol��2����2�֣� d����1�֣�
��4���ٸ�����1�֣� ��CH3OH �� 6e��+8OH��= CO32��+6H2O����2�֣�
��1�����ݸ�˹���ɢ�+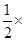 �ڡ�
�ڡ�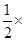 �ۿ�֪��CO(g) + 2H2(g)
�ۿ�֪��CO(g) + 2H2(g)  CH3OH(g) ��H����H1+
CH3OH(g) ��H����H1+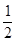 ��H2 ��
��H2 ��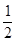 ��H3
��H3
��2����ͼʾ��֪������ͬ��ѹǿ�£������¶�ʱCO��ת���ʽ��ͣ�������ƽ�����ƣ�����Ӧ�Ƿ��ȷ�Ӧ����H<0
��Ե�����������߲��ʣ�����1.3��104kPa�£�CO��ת�����Ѿ��ܸߣ��������ѹǿCO��ת������߲��������ɱ����ӣ��ò���ʧ����ѡ������Ϊ250�桢1.3��104kPa
��3����Ӧ��CH3OH(g) CO(g) + 2H2(g)
CO(g) + 2H2(g)
��n�� 1 0 0
��n�� 0.5 0.5 1
ƽ��n�� 0.5 0.5 1
v(CH3OH)=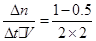 = 0.125mol��L��1�� min��1��
= 0.125mol��L��1�� min��1��
���¶��£�CO(g) + 2H2(g) CH3OH(g)��ƽ�ⳣ��K=
CH3OH(g)��ƽ�ⳣ��K=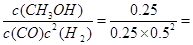 4 L2��mol��2��
4 L2��mol��2��
���ݡ���������£�����ʼ����CH3OH(g)�����ʵ�����ԭ����2����
a��ƽ�ⳣ��ֻ���¶��йأ��Ƕ�ֵ��
b���൱�ڼ�ѹ��CH3OH��ƽ��Ũ�Ƚ�����ԭƽ��Ũ�ȵ�����
c�����ӷ�Ӧ��Ũ�ȣ���Ӧ���ʼӿ죬�ﵽƽ���ʱ�佫��С����û�ж�����ϵ
d������������Ϊԭ�ȵ���������ƽ��ʱ������ܶ�Ϊԭ�ȵ���������������
��4��ȼ�ϵ����ͨ��������һ���õ��ӣ�Ϊ��������ȼ�ϼ״�ʧ���ӣ�������
����ܷ�ӦΪ��2CH3OH��3O2+4OH��=6H2O+2 CO32��
������ӦΪ��O2��4e����2H2O=4OH��
�ܷ�Ӧ��ȥ������Ӧ�õ�������Ӧ��CH3OH �� 6e��+8OH��= CO32��+6H2O
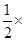 �ڡ�
�ڡ�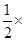 �ۿ�֪��CO(g) + 2H2(g)
�ۿ�֪��CO(g) + 2H2(g)  CH3OH(g) ��H����H1+
CH3OH(g) ��H����H1+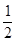 ��H2 ��
��H2 ��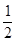 ��H3
��H3��2����ͼʾ��֪������ͬ��ѹǿ�£������¶�ʱCO��ת���ʽ��ͣ�������ƽ�����ƣ�����Ӧ�Ƿ��ȷ�Ӧ����H<0
��Ե�����������߲��ʣ�����1.3��104kPa�£�CO��ת�����Ѿ��ܸߣ��������ѹǿCO��ת������߲��������ɱ����ӣ��ò���ʧ����ѡ������Ϊ250�桢1.3��104kPa
��3����Ӧ��CH3OH(g)
 CO(g) + 2H2(g)
CO(g) + 2H2(g)��n�� 1 0 0
��n�� 0.5 0.5 1
ƽ��n�� 0.5 0.5 1
v(CH3OH)=
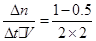 = 0.125mol��L��1�� min��1��
= 0.125mol��L��1�� min��1�����¶��£�CO(g) + 2H2(g)
 CH3OH(g)��ƽ�ⳣ��K=
CH3OH(g)��ƽ�ⳣ��K=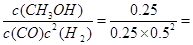 4 L2��mol��2��
4 L2��mol��2�����ݡ���������£�����ʼ����CH3OH(g)�����ʵ�����ԭ����2����
a��ƽ�ⳣ��ֻ���¶��йأ��Ƕ�ֵ��
b���൱�ڼ�ѹ��CH3OH��ƽ��Ũ�Ƚ�����ԭƽ��Ũ�ȵ�����
c�����ӷ�Ӧ��Ũ�ȣ���Ӧ���ʼӿ죬�ﵽƽ���ʱ�佫��С����û�ж�����ϵ
d������������Ϊԭ�ȵ���������ƽ��ʱ������ܶ�Ϊԭ�ȵ���������������
��4��ȼ�ϵ����ͨ��������һ���õ��ӣ�Ϊ��������ȼ�ϼ״�ʧ���ӣ�������
����ܷ�ӦΪ��2CH3OH��3O2+4OH��=6H2O+2 CO32��
������ӦΪ��O2��4e����2H2O=4OH��
�ܷ�Ӧ��ȥ������Ӧ�õ�������Ӧ��CH3OH �� 6e��+8OH��= CO32��+6H2O

��ϰ��ϵ�д�
 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
�����Ŀ
 Ҫ���ڣ����������Ҫ�����Ƿ�ֹ(����)
Ҫ���ڣ����������Ҫ�����Ƿ�ֹ(����) �ն����� ������ЧӦ �ݰ�ɫ��Ⱦ
�ն����� ������ЧӦ �ݰ�ɫ��Ⱦ