��Ŀ����
Ŀǰ������β���ѳ�Ϊ�������п�������Ҫ��Ⱦ������β���к���CO��NO�ȶ�����Ⱦ�
������ȼ����һ�㲻������β����������NO�����Ļ�ѧ����ʽΪ__ ___ ____��
�� ��������β����NO��CO��һ�ַ����ǣ�����������������װ��һ����ת��װ�ã�ʹNO��CO��Ӧ������CO2�� N2����Ӧ�Ļ�ѧ����ʽΪ ___ _______��
�� ���д�ʩ�У��ܼ��ٻ��������β����Ⱦ��Ч�ҿ��е���__ ___
�� �ƶ��ϸ��β���ŷű������ϸ�ִ�С�
�� ���������Դ��������������̫���������ȡ�
�� ������д�������ߡ�
�� ��������ƾӳ��н�����
������ȼ����һ�㲻������β����������NO�����Ļ�ѧ����ʽΪ__ ___ ____��
�� ��������β����NO��CO��һ�ַ����ǣ�����������������װ��һ����ת��װ�ã�ʹNO��CO��Ӧ������CO2�� N2����Ӧ�Ļ�ѧ����ʽΪ ___ _______��
�� ���д�ʩ�У��ܼ��ٻ��������β����Ⱦ��Ч�ҿ��е���__ ___
�� �ƶ��ϸ��β���ŷű������ϸ�ִ�С�
�� ���������Դ��������������̫���������ȡ�
�� ������д�������ߡ�
�� ��������ƾӳ��н�����
��N2 + O2 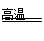 2NO��
2NO��
�� 2NO + 2CO N2 + 2CO2���� �٢ڡ�
N2 + 2CO2���� �٢ڡ�
 2NO��
2NO���� 2NO + 2CO
 N2 + 2CO2���� �٢ڡ�
N2 + 2CO2���� �٢ڡ�����������ʱ��������£��ڸ�������N2��O2������Ӧ�� N2 + O2  2NO��
2NO��
��������֪��Ӧ��Ͳ��P������2NO + 2CO N2 + 2CO2��
N2 + 2CO2��
�Ǵ�����������ƾ��Dz����ܵģ�ֻ�ܼ����ŷŻ�Ѱ������Դ�������ѡ�٢ڡ�
 2NO��
2NO����������֪��Ӧ��Ͳ��P������2NO + 2CO
 N2 + 2CO2��
N2 + 2CO2���Ǵ�����������ƾ��Dz����ܵģ�ֻ�ܼ����ŷŻ�Ѱ������Դ�������ѡ�٢ڡ�

��ϰ��ϵ�д�
�����Ŀ
 CH3OH(g)�ġ�H�� ��
CH3OH(g)�ġ�H�� ��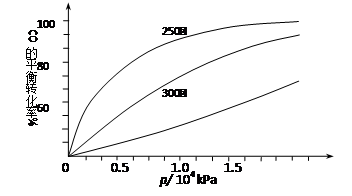


 ����Ϳ�ϻ�����
����Ϳ�ϻ�����