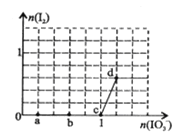
��Ŀ����
����Ŀ����֪���� Fe2O3(s)��3C(ʯī)=2Fe(s)��3CO(g) ��H����489.0 kJ��mol��1
�� CO(g)��![]() O2(g)=CO2(g) ��H����283.0 kJ��mol��1
O2(g)=CO2(g) ��H����283.0 kJ��mol��1
�� C(ʯī)��O2(g)=CO2(g) ��H����393.5 kJ��mol��1
��4Fe(s)��3O2(g)=2Fe2O3(s)����H�� ��
A����1 164.1 kJ��mol��1 B����1 641.0 kJ��mol��1
C����259.7 kJ��mol��1 D����519.4 kJ��mol��1
���𰸡�B
��������
�������������Ŀ�귴Ӧ����ʽ��6������2������6�������ó���4Fe(s)��3O2(g)=2Fe2O3(s) ��H=��1641.0kJ��mol��1����ѡ��B��ȷ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ