��Ŀ����
�����ĩX��Fe2O3��CuO��ɣ�ij�о���ѧϰС��ѡ����ͼ��װ�ã��г�װ������ȥ��ʵ��ǰ���Ѽ��װ�������ԣ�̽��X�����̿�۷�����Ӧʱ��������CO2�⣬�Ƿ�������������
��ش�
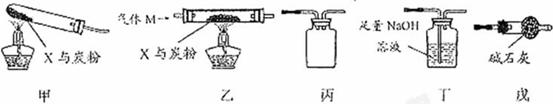
��1�������ס���������˳������װ�ã���____________________������ͼװ�ô��ţ�װ�ú��Լ����������ʼ��Ӧ�����徭������պ������ݷ��֣�����̼����Ԫ�ص�������������CO2�����������ܵ�ԭ���������CO2���������������������_____________.
��2��Ϊʹʵ���õ����ݸ���ȷ˵�����⣬һЩͬѧ���ҡ��������˳������װ�ý���ʵ�飬���Ҽ���ǰ��ͨһ������M��ֹͣ���Ⱥ���ͨһ��������塣
������M������____________ ������ĸ����
a��CO2 b��H2 c��N2 d��CO e��O2
��ֹͣ���Ⱥ���ͨһ������M��ԭ����_____________________________________��
��3����������װ�þ����ڵ�ͬ��������ȱ����________________________________��
��4����С��ͬѧ���ʵ�飨��ͼ����X����ת����ÿһ������Ӧ��ȫ��
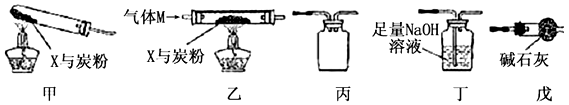
��1�������ס���������˳������װ�ã���____________________������ͼװ�ô��ţ�װ�ú��Լ����������ʼ��Ӧ�����徭������պ������ݷ��֣�����̼����Ԫ�ص�������������CO2�����������ܵ�ԭ���������CO2���������������������_____________.
��2��Ϊʹʵ���õ����ݸ���ȷ˵�����⣬һЩͬѧ���ҡ��������˳������װ�ý���ʵ�飬���Ҽ���ǰ��ͨһ������M��ֹͣ���Ⱥ���ͨһ��������塣
������M������____________ ������ĸ����
a��CO2 b��H2 c��N2 d��CO e��O2
��ֹͣ���Ⱥ���ͨһ������M��ԭ����_____________________________________��
��3����������װ�þ����ڵ�ͬ��������ȱ����________________________________��
��4����С��ͬѧ���ʵ�飨��ͼ����X����ת����ÿһ������Ӧ��ȫ��

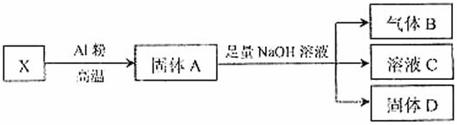
����������B�Ļ�ѧ����ʽ��________________________________��
�ڽ�����DͶ�����ϡ���Ტ��ַ�Ӧ���ˣ�������Һ����˫��ˮ���û�ɫ��Һ�� д��������˫��ˮʱ��Һ�з�����Ӧ�����ӷ���ʽ�� _________________________��
������֪����X������Ϊ7.2g��Ӧ��AI�۵�������2.7g������B�ڱ�״���µ����Ϊ672ml�������D��������_________g��
�ڽ�����DͶ�����ϡ���Ტ��ַ�Ӧ���ˣ�������Һ����˫��ˮ���û�ɫ��Һ�� д��������˫��ˮʱ��Һ�з�����Ӧ�����ӷ���ʽ�� _________________________��
������֪����X������Ϊ7.2g��Ӧ��AI�۵�������2.7g������B�ڱ�״���µ����Ϊ672ml�������D��������_________g��
��1���ס��� ����CO2���������װ����
��2����c���ڽ�װ���в�����CO2���崵������ֹ��Һ��������
��3����β������װ��
��4����2Al+2NaOH+2H2O=2NaAlO2+3H2������2Fe2++H2O2+2H+=2Fe2++2H2O����5.28
��2����c���ڽ�װ���в�����CO2���崵������ֹ��Һ��������
��3����β������װ��
��4����2Al+2NaOH+2H2O=2NaAlO2+3H2������2Fe2++H2O2+2H+=2Fe2++2H2O����5.28

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�����ĩX�п��ܺ���FeO��Fe2O3��MnO2��K2SiO3��K2SO3��KAlO2��MgCl2��K2CO3��NaNO2�е������֣�Ϊȷ���ù����ĩ�ijɷ֣���ȡX��������ʵ�飬ʵ����̼��������£�
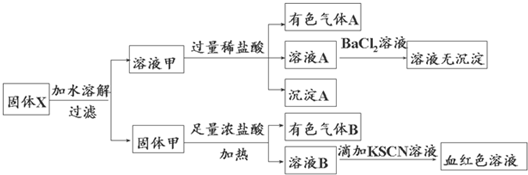
��������ʵ�飬����˵����ȷ���ǣ�������

��������ʵ�飬����˵����ȷ���ǣ�������
| A����Һ����һ������K2SiO3��NaNO2�����ܺ���KAlO2��K2CO3 | B����������Һ���м�������ϡ������ټ���BaCl2��Һ��������ж���Һ���Ƿ���K2SO3 | C������A������Bһ����Ϊ������ | D��ԭ�������һ������Fe2O3 |