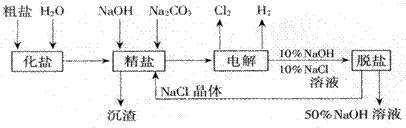
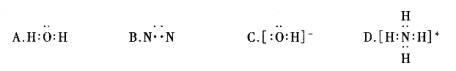��Ŀ����
(1998���Ϻ���31)�ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ14-4����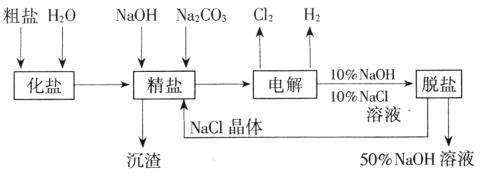
ͼ14-4
������ͼ�����������գ�
(1)�ڵ������У����Դ���������ĵ缫����������Ӧ�Ļ�ѧ����ʽΪ �����Դ���������ĵ缫��������ҺpH (ѡ����䡱�����ߡ����½���)��
(2)��ҵʳ�κ�Ca2+��Mg2+�����ʡ����ƹ��̷�����Ӧ�����ӷ���ʽΪ
�� ��
(3)���������![]() �����ϸߣ��������ӱ��Լ���ȥ
�����ϸߣ��������ӱ��Լ���ȥ![]() ���ñ��Լ������� (ѡ��a��b��c��ѡ�۷�)��
���ñ��Լ������� (ѡ��a��b��c��ѡ�۷�)��
a.Ba(OH)2 b.Ba(NO3)2 c.BaCl2
(4)Ϊ��Ч��ȥCa2+��Mg2+��![]() �������Լ��ĺ���˳��Ϊ (ѡ��a��b��c��ѡ�۷�)��
�������Լ��ĺ���˳��Ϊ (ѡ��a��b��c��ѡ�۷�)��
a.�ȼ�NaOH�����Na2CO3���ټӱ��Լ�
b.�ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
c.�ȼӱ��Լ������NaOH���ټ�Na2CO3
(5)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ�� ����ȴ�� (��д��������)��ȥNaCl
(6)�ڸ�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��������Ĥ������ʳ��ˮʱ��Cl2��NaOH��ֽӴ����������NaClO��H2����Ӧ�Ļ�ѧ����ʽΪ ��
�𰸣�
(1)2Cl����2e��====Cl2������
(2)Ca2++![]() ====CaCO3��
====CaCO3��
Mg2++2OH��====Mg(OH)2��
(3)a��c (4)b��c (5)����������
(6)NaCl+H2O====NaClO+H2��
��2NaCl+2H2O====H2��+Cl2��+2NaOH
Cl2+2NaOH====NaCl+NaClO+H2O