��Ŀ����
(1998���Ϻ���35)ʯ�ͼ����������ڹ�������ʱȼ�գ���������CO����Ҳ�dz����Ĵ�����Ⱦ��֮һ����ij��̬����5������Ĵ�����ȼ�գ�����ͨ������Na2O2���ڵ�����������³�ַ�Ӧ�����ɵ����������С��ȼ�պ���������3/8(�����������100 �����ϣ�1.01��105Paʱ�ⶨ)��(1)��д��ͨʽΪCxHy��ij���ڹ�������ʱ��ȼ�յĻ�ѧ����ʽ(CO2��ϵ���趨Ϊm)��
(2)��m=2ʱ��������ķ���ʽ��
(3)��1 molij��̬���ڹ�������ʱȼ�գ�����������Na2O2�͵������������
����3 mol�������ҹ���Na2O2���ط�ΧΪ90g�ܦ�����118g���������ܵķ���ʽ��ȼ�ղ���CO��CO2�����ʵ���֮�ȣ�����������±�
���ķ���ʽ | n��CO��/n��CO2�� |
|
|
�𰸣�
��1��CxHy+![]() O2��mCO1+
O2��mCO1+![]() H2O+(x-m)CO
H2O+(x-m)CO
(2)

��m=2ʱ����ã�x=4 y=8 ���Ը���ΪC4H8
(3)
����ʽ |
|
C3H8 |
|
C4H6 |
|
���¼�������۹��̹��ο���
��������������
������������CO2��+��W��H2O��+��W��CO����28��+y+28(x-m)��28x+y
�ٸ���Na2O2���� 90��28x+y��118���۵���̬����x=3ʱy��6��x=4ʱ����6��
�������º����Ĵ��У�C4H6��C4H4��C3H8��C3H6
�ڸ���ȼ�ղ���ͨ������Na2O2����O2���������3���
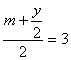 ��Ϊx��m ��������6-
��Ϊx��m ��������6-![]()
���۵���̬����x=3ʱ y��6��x=4ʱ y��4
���ݢ٢�������ͬʱ���㣬�Ӷ���C4H4����C3H6��
���Ը����Ŀ�����ΪC3H8��C4H6��
�ٸ���![]() ��3��
��3��
y=8ʱ��m=2 ![]() =
=![]()
y=6ʱ��m=3 ![]() =
=![]()



