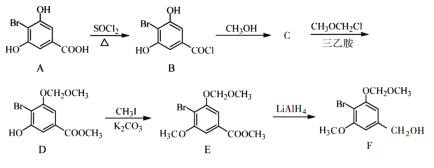
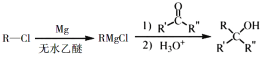ћвƒњƒЏ»Ё
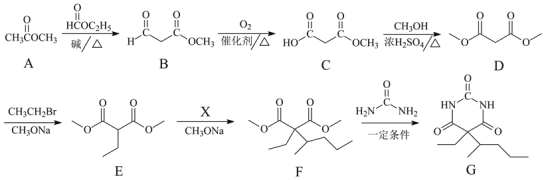
°Њћвƒњ°њїѓ—Іјі‘і”Џ…ъїо”÷Јюќс”Џ…ъїо£ђ«л”√їѓ—І”√”пїЎірѕ¬Ѕ–ќ ћв£Ї
£®1£©∆ѓЈџЊЂ÷–”––І≥…Ј÷µƒїѓ—І љќ™£Ї___________
£®2£©°∞єи≤ƒЅѕ°± «ќёїъЈ«љр ф≤ƒЅѕµƒ÷чљ«£ђ∆д÷–єгЈЇ”¶”√”ЏєвµЉѕЋќђµƒ≤ƒЅѕ «£Ї____________
£®3£©ѕтїл„«µƒЋЃ÷–Љ”»л√чЈѓKAl(SO4)2°§12H2OЇу£ђЋЃњ…µ√µљЊїїѓ°£–і≥ц√чЈѓ‘ЏЋЃ÷–µƒµзјлЈљ≥ћ љ£Ї___________°£Ќщ√чЈѓ»№“Ї÷–÷рµќЉ”»лBa(OH)2»№“Ї£ђ»ф єSO42- «°Ї√Ќк»Ђ≥Ѕµн£ђЈҐ…ъЈі”¶µƒјл„”Јљ≥ћ љќ™£Ї___________£ї»ф єAl3+«°Ї√Ќк»Ђ≥Ѕµн£ђЈҐ…ъЈі”¶µƒјл„”Јљ≥ћ љќ™£Ї_____°£
£®4£©“ы”√ЋЃ÷–µƒNO3-ґ‘»Ћјаљ°њµ≤ъ…ъќ£Ї¶£ђќ™ЅЋљµµЌ“ы”√ЋЃ÷–NO3-µƒ≈®ґ»£ђњ…“‘‘ЏЉо–‘ћхЉюѕ¬”√¬ЅЈџљЂNO3-їє‘≠ќ™N2£ђ∆дїѓ—ІЈљ≥ћ љќ™£Ї 10Al + 6NaNO3 + 4NaOH = 10NaAlO2 + 3N2°ь + 2H2O
«лїЎірѕ¬Ѕ–ќ ћв£Ї
Ґў‘Џїѓ—ІЈљ≥ћ љ…ѕ”√µ•ѕя«≈±к≥цЄ√Јі”¶÷–µз„”„™“∆µƒЈљѕтЇЌ эƒњ£Ї____________
ҐЏ…ѕ цЈі”¶÷–»ф…ъ≥…±к„Љ„іњцѕ¬3.36LN2£ђ‘т„™“∆µƒµз„” эƒњќ™£Ї_____________°£
°Њір∞Є°њCa(ClO)2 SiO2 KAl(SO4)2 = K+ + Al3+ + 2SO42- 2SO42- +2Ba2++Al3++4OH- = 2BaSO4°э + AlO2-+ 2H2O 2Al3+ + 3Ba2+ + 6OH- + 3SO42- = 2Al(OH)3°э + 3BaSO4°э ![]() 1.5NA
1.5NA
°Њљвќц°њ
јл„”Јљ≥ћ љ й–і ±£ђ“‘…ўЅњµƒќп÷ ÷–јл„”≈®ґ»ќ™Јі”¶ЇЋ–ƒ£ђљш––…ъ≥…ќп≥Ѕµнїт∆шћеµƒ й–і£їЄщЊЁ—хїѓїє‘≠Јі”¶‘≠јн й–іµ•Љь«≈£ђЉэЌЈі”їє‘≠ЉЅ÷Єѕт—хїѓЉЅ°£
£®1£©∆ѓ∞„ЊЂ «”√Cl2”лCa(OH)2Јі”¶÷∆µ√µƒ£ђ∆д÷ч“™≥…Ј÷ «CaCl2ЇЌCa(ClO)2£ђ”––І≥…Ј÷ «Ca(ClO)2°£
£®2£©євµЉѕЋќђ÷ч“™≥…Ј÷ «SiO2°£
£®3£©√чЈѓќ™«њµзљв÷ £ђ‘ЏЋЃ÷–µзјлµ√µљAl3+°ҐK+°ҐSO42-£ђµзјлЈљ≥ћ љќ™KAl(SO4)2=K++Al3++2SO42-£ї»ф єSO42- «°Ї√Ќк»Ђ≥Ѕµн£ђ…иKAl(SO4)2ќ™1mol£ђЉ”»лµƒBa(OH)2ќ™2mol£ђ”лAl3+Јі”¶«°Ї√…ъ≥…AlO2-£ђЈҐ…ъЈі”¶µƒјл„”Јљ≥ћ љќ™2SO42- +2Ba2++Al3++4OH- = 2BaSO4°э + AlO2- + 2H2O£їЉ”»лBa(OH)2÷ЅAl3+«°Ї√Ќк»Ђ≥Ѕµн ±£ђ…иKAl(SO4)2ќ™1mol£ђ–и“™OH-3mol£ђ‘тBa2+ќ™1.5mol£ђ…ъ≥…BaSO4 ќ™1.5mol£ђ»№“Ї÷–їє”–0.5molSO42-√ї”–≤ќЉ”Јі”¶£ђЈі”¶јл„”Јљ≥ћ љќ™2Al3++3SO42-+3Ba2++6OH-=2Al(OH)3°э+3BaSO4°э°£
£®4£©Є√Јі”¶÷–Al‘™ЋЎі”0Љџ…эЄяµљ+3Љџ£ђ І»•µз„” э «3°Ѕ10=30£ђNaNO3÷–+5ЉџN‘™ЋЎїѓЇѕЉџљµµЌµљ0Љџ…ъ≥…N2£ђ![]() °£…ъ≥…3molN2£ђ„™“∆30molµз„”£ђЋщ“‘µ±N2ќ™0.15mol ±£ђ„™“∆µз„” эќ™1.5NA°£
°£…ъ≥…3molN2£ђ„™“∆30molµз„”£ђЋщ“‘µ±N2ќ™0.15mol ±£ђ„™“∆µз„” эќ™1.5NA°£

 ћмћмѕт…ѕ“ї±ЊЇ√ЊнѕµЅ–ір∞Є
ћмћмѕт…ѕ“ї±ЊЇ√ЊнѕµЅ–ір∞Є –°—І…ъ10Ј÷÷””¶”√ћвѕµЅ–ір∞Є
–°—І…ъ10Ј÷÷””¶”√ћвѕµЅ–ір∞Є°Њћвƒњ°њѕ¬Ѕ– µ—й≤ў„ч°Ґѕ÷ѕуЇЌљб¬џґЉ’э»Јµƒ « £® £©
—°ѕо | µ—й≤ў„ч | ѕ÷ѕу | љб¬џ |
A | љЂ ѓјѓ”ЌЈ÷љв≤ъ…ъµƒ∆шћеЌ®»лЋб–‘Єя√ћЋбЉЎ»№“Ї÷– | „ѕЇм…Ђ»№“Ї±дќё…Ђ | ѓјѓ”ЌЅ—љв“їґ®≤ъ…ъЅЋ““ѕ© |
B | ”√ЋЃљюєэµƒ√ёї®∞ьєьћъЈџЈ≈»л ‘є№≤ҐЉ”»»£ђљЂ∆шћеЌ®»лЋƒ¬»їѓћЉ»№“Ї÷– | Ћƒ¬»їѓћЉ÷–≤ъ…ъ∆ш≈Ё | ћъЈџ”лЋЃ’ф∆ш‘ЏЄяќ¬ѕ¬“їґ®≤ъ…ъЅЋ«в∆ш |
C | ѕт÷ЎЄхЋбЉЎ»№“Ї÷–µќЉ”““іЉ | ≥»Їм…Ђ»№“Ї±д¬ћ…Ђ | ““іЉ±нѕ÷≥цїє‘≠–‘ |
D | ѕт2ml0.1mol/LµƒAgNO3»№“Ї÷–ѕ»µќЉ”2µќ0.1mol/L NaCl»№“Ї£ђ‘ўµќ»л | ѕ»≤ъ…ъ∞„…Ђ≥Ѕµн£ђЇу≥Ѕµн±д≥…ї∆…Ђ | ѕаЌђќ¬ґ»ѕ¬£ђAgCl‘ЏЋЃ÷–µƒ»№љвґ»іу”ЏAgIµƒ»№љвґ» |
A.AB.BC.CD.D
°Њћвƒњ°њ“їґ®ќ¬ґ» ±£ђѕт2.0 LЇг»Ё√№±’»Ё∆ч÷–≥д»л1.0 mol PCl5£ђЈҐ…ъЈі”¶£Ї PCl5(g) ![]() Cl2(g)+PCl3(g)Њ≠“їґќ ±ЉдЇуЈі”¶іпµљ∆љЇв°£Јі”¶єэ≥ћ÷–≤вµ√µƒ≤њЈ÷ эЊЁЉыѕ¬±н£Ї
Cl2(g)+PCl3(g)Њ≠“їґќ ±ЉдЇуЈі”¶іпµљ∆љЇв°£Јі”¶єэ≥ћ÷–≤вµ√µƒ≤њЈ÷ эЊЁЉыѕ¬±н£Ї
Јі”¶ ±Љд/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3)/mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
£®1£©ƒ№±н√чЄ√Јі”¶іпµљ∆љЇв„іћђµƒ «________£®ћо–тЇ≈£©£ї
A£Ѓ…ъ≥…1molPCl3µƒЌђ ±…ъ≥…1molPCl5 B£ЃїмЇѕ∆шћеµƒ∆љЊщѕаґ‘Ј÷„”÷ Ѕњ≤ї±д
C£ЃPCl5°ҐCl2°ҐPCl3»э’яµƒ≈®ґ»±»÷µ1£Ї1£Ї1 D£ЃїмЇѕ∆шћеµƒ√№ґ»≤ї±д
£®2£©…ѕ цќ¬ґ»ѕ¬£ђіпµљ∆љЇв ±£ђPCl5µƒ„™їѓ¬ ќ™____£їЄ√Јі”¶µƒ∆љЇв≥£ эK= ___°£
£®3£©Јі”¶‘Џ«∞50 sƒЏµƒ∆љЊщЋў¬ ќ™v(PCl3)=_____°£
£®4£©±£≥÷∆дЋыћхЉю≤ї±д£ђ»ф…эЄяќ¬ґ»£ђЈі”¶÷Ў–¬іпµљ∆љЇв£ђ∆љЇв ±c(PCl3)=0.11moI°§L£≠l£ђ‘т’эЈі”¶µƒ¶§H__0£®ћо°∞>°±їт°∞<°±£©°£
£®5£©ѕаЌђќ¬ґ»ѕ¬£ђ»ф∆р Љ ±ѕт»Ё∆ч÷–≥д»л1.0 molPCl5°Ґ0.20mol PCl3ЇЌ0.20 mol Cl2£ђ‘тЈі”¶іпµљ∆љЇв«∞v(’э) __ v(ƒж)£®ћо°∞>°±їт°∞<°±£©