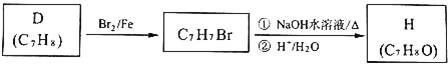��Ŀ����
����Ŀ����ŵ����(fenofibrate)�ǽ���ͬ��������������ҩ�����һ���ϳ�·�����£�
��֪�����������л�����Һ���������������£�����![]() ȡ����
ȡ����
![]()
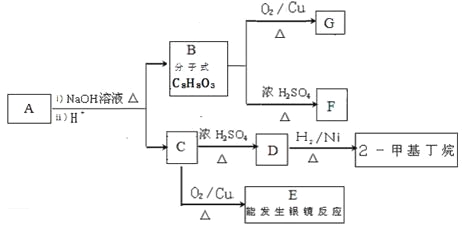
��1��B������Ϊ_______________��
��2��C���������ŵ�����Ϊ___________��
��3��д���������ʵĽṹ��ʽb_____________��F__________________��
��4��д��G��H�ķ�Ӧ����ʽ_______________��
��5��д��ͬʱ��������������D��ͬ���칹��ṹͲʽ____________��
���ܷ���������Ӧ������5�ֲ�ͬ����������������л��
1 mol���л����������NaOH�����ʵ���Ϊ_______________��
��6����2-����ϩΪԭ���Ʊ�E����ƺϳ�·��(�����Լ���ѡ)��
___________________________________________________
���𰸡����ȼױ���4-�ȼױ� ��ԭ�ӡ��Ȼ� ![]()
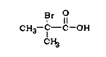
![]()
![]() 4mol
4mol 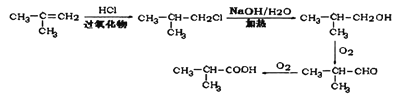
��������
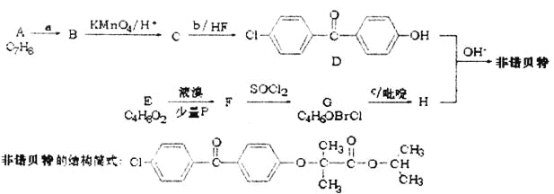
��D���ŵ���صĽṹ��ʽ����֪HΪ![]() ����ת����ϵ����Ӧ��Ϣ����֪EΪ��CH3��2CHCOOH��E���巢��ȡ����Ӧ����FΪ
����ת����ϵ����Ӧ��Ϣ����֪EΪ��CH3��2CHCOOH��E���巢��ȡ����Ӧ����FΪ![]() ��F��SO2Cl����ȡ����Ӧ����GΪ
��F��SO2Cl����ȡ����Ӧ����GΪ![]() ���Ա�G��H�Ľṹ��֪cΪ
���Ա�G��H�Ľṹ��֪cΪ![]() ����D�Ľṹ�����A�ķ���ʽ��֪AΪ
����D�Ľṹ�����A�ķ���ʽ��֪AΪ![]() ����������������λȡ����Ӧ����BΪ
����������������λȡ����Ӧ����BΪ![]() ��B�����Ը��������Һ��������CΪ
��B�����Ը��������Һ��������CΪ![]() ������֪bΪ
������֪bΪ![]() ����6����2-����ϩ��HCl�����ӳɷ�Ӧ�õ�
����6����2-����ϩ��HCl�����ӳɷ�Ӧ�õ�![]() ���ٷ���ˮ�ⷴӦ�õ�
���ٷ���ˮ�ⷴӦ�õ�![]() �������������õ�
�������������õ�![]() ���������õ�E��
���������õ�E��
��D���ŵ���صĽṹ��ʽ����֪HΪ![]() ����ת����ϵ����Ӧ��Ϣ����֪EΪ��CH3��2CHCOOH��E���巢��ȡ����Ӧ����FΪ
����ת����ϵ����Ӧ��Ϣ����֪EΪ��CH3��2CHCOOH��E���巢��ȡ����Ӧ����FΪ![]() ��F��SO2Cl����ȡ����Ӧ����GΪ
��F��SO2Cl����ȡ����Ӧ����GΪ![]() ���Ա�G��H�Ľṹ��֪cΪ
���Ա�G��H�Ľṹ��֪cΪ![]() ����D�Ľṹ�����A�ķ���ʽ��֪AΪ
����D�Ľṹ�����A�ķ���ʽ��֪AΪ![]() ����������������λȡ����Ӧ����BΪ
����������������λȡ����Ӧ����BΪ![]() ��B�����Ը��������Һ��������CΪ
��B�����Ը��������Һ��������CΪ![]() ������֪bΪ
������֪bΪ![]() ��
��
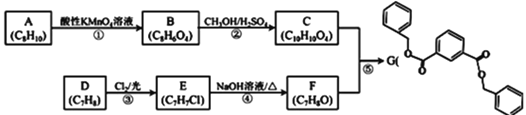
��1��BΪ![]() ������Ϊ4-�ȼױ����ʴ�Ϊ��4-�ȼױ���
������Ϊ4-�ȼױ����ʴ�Ϊ��4-�ȼױ���
��2��CΪ![]() ������������Ϊ����ԭ�ӡ��Ȼ����ʴ�Ϊ����ԭ�ӡ��Ȼ���
������������Ϊ����ԭ�ӡ��Ȼ����ʴ�Ϊ����ԭ�ӡ��Ȼ���
��3��b�Ľṹ��ʽΪ![]() ��F�Ľṹ��ʽΪ��
��F�Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��![]() ��
��
��4��G��H�ķ�Ӧ����ʽ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
��5��ͬʱ��������������D��ͬ���칹�壺���ܷ���������Ӧ��˵������ȩ�����ں�5�ֲ�ͬ����������������л�����м�������γɵ���������ͬ���칹��ṹ��ʽΪ��![]() ��±����ˮ��õ����ǻ���HCl����������2mol NaOH������ˮ��õ����ǻ����Ȼ�����������2mol NaOH��1mol���л����������NaOH�����ʵ���Ϊ4mol��
��±����ˮ��õ����ǻ���HCl����������2mol NaOH������ˮ��õ����ǻ����Ȼ�����������2mol NaOH��1mol���л����������NaOH�����ʵ���Ϊ4mol��
�ʴ�Ϊ��![]() ��4mol��
��4mol��
��6��2-����ϩ��HCl�����ӳɷ�Ӧ�õ�![]() ���ٷ���ˮ�ⷴӦ�õ�
���ٷ���ˮ�ⷴӦ�õ�![]() �������������õ�
�������������õ�![]() ���������õ�E���ϳ�·������ͼΪ��
���������õ�E���ϳ�·������ͼΪ��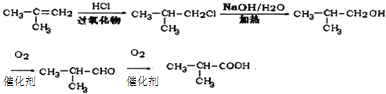 ��
��

����Ŀ���±��Ǽס��ҡ����������������л�����й���Ϣ��
�� | ����ʹ������Ȼ�̼��Һ��ɫ���ڱ���ģ��Ϊ |
�� | ����C��H����Ԫ����ɣ������ģ��Ϊ |
�� | ����C��H��O����Ԫ����ɣ�������Na��Ӧ���������� |
�� | ����Է��������ȱ��٣������ɱ��������� |
�� | ����C��H��O����Ԫ����ɣ������ģ��Ϊ |
�ش��������⣺
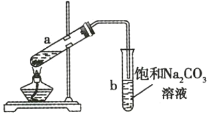
(1)����������Ȼ�̼��Һ��Ӧ���ò����������___________________��
(2)�������������ӳɷ�Ӧ���������ʼ����ڼ��ڷ�����ɺͽṹ�����Ƶ��л�����һ���ࣨ�׳ơ�ͬϵ��������Ǿ�����ͨʽ![]() ����
����![]() ___________________ʱ�������л��↑ʼ����ͬ���칹�塣
___________________ʱ�������л��↑ʼ����ͬ���칹�塣
(3)�Ҿ��е�������___________________������ţ���
����ɫ��ζҺ�� ���ж� �۲�����ˮ ���ܶȱ�ˮ�� ��������![]() ��Һ����ˮ��Ӧ��ʹ����ɫ ���κ������¶�����������Ӧ
��Һ����ˮ��Ӧ��ʹ����ɫ ���κ������¶�����������Ӧ
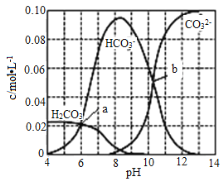
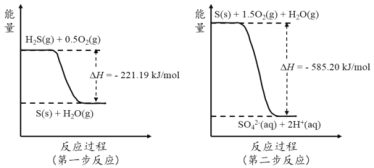
(4)�����췴Ӧ��������Է�������Ϊ100��������������Ӧ�ķ�Ӧ����Ϊ_____________________����Ӧ�Ļ�ѧ����ʽΪ_______________________��
(5)д�����������ɶ��Ļ�ѧ��Ӧ����ʽ��__________________��