��Ŀ����
��֪��25�桢l0lkPa������
4Al(s)��3O2(g)=2Al2O3(s) ��H=-2834.9kJ/mol
4Al(s)��2O3(g)=2Al2O3(s) ��H=-3119.91kJ/mol: �ɴ˵ó��Ľ�����ȷ����
| A����������O2��O3�����ͣ���O2��O3Ϊ���ȷ�Ӧ |
| B����������O2��O3�����ͣ���O2��O3Ϊ���ȷ�Ӧ |
| C��O3��O2�ȶ�����O2��O3Ϊ���ȷ�Ӧ |
| D��O2��O3�ȶ�����O2��O3Ϊ���ȷ�Ӧ |
A
��������������ɸ����������Ȼ�ѧ����ʽ��������ͬ�����µõ���ͬ����������������������������Ҫ�ࡣ˵����������O3��O2�����ߡ�����Խ��Խ�ȶ����ɳ���������Ϊ���ȷ�Ӧ����ȷѡ��ΪA��
���㣺����������������ȶ��Լ�������������С��֪ʶ��

��֪��Ӧ��H2S(g)��aO2(g)=X��cH2O(l)����H������H��ʾ�÷�Ӧ�ı�ȼ���ȣ���XΪ (����)
| A��S(s) | B��SO2(g) | C��SO3(g) | D��SO3(l) |
25�桢101 kPa�£�̼������������������ǵ�ȼ����������393.5 kJ/mol��285.8 kJ/mol��890.3 kJ/mol��2 800 kJ/mol���������Ȼ�ѧ����ʽ��ȷ���� ( )
A��C(s)��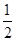 O2(g)=CO(g) ��H����393.5 kJ/mol O2(g)=CO(g) ��H����393.5 kJ/mol |
| B��2H2(g)��O2(g)=2H2O(g) ��H����571.6 kJ/mol |
C��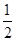 C6H12O6(s)��3O2(g)=3CO2(g)��3H2O(l)��H����1 400 kJ/mol C6H12O6(s)��3O2(g)=3CO2(g)��3H2O(l)��H����1 400 kJ/mol |
| D��CH4(g)��2O2(g)=CO2(g)��2H2O(g)��H����890.3 kJ/mol |
����ͼ��ʾ����H1=��393.5 kJ?mol-1����H2=��395.4 kJ?mol-1������˵�����ʾʽ��ȷ����( )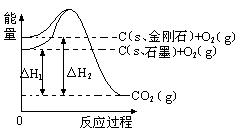
| A��C��s��ʯī��=C��s�����ʯ�� ��H="+1.9" kJ?mol-1 |
| B��ʯī�ͽ��ʯ��ת���������仯 |
| C�����ʯ���ȶ���ǿ��ʯī |
| D��1 molʯī���ܼ��ܱ�1 mol���ʯ���ܼ���С1.9 kJ |
�����Ȼ�ѧ����ʽ�У���ȷ����
| A�������ȼ���ȡ�H = -890��3kJ/mol�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g)�T CO2(g)+2H2O(g)��H = -890��3kJ/mol |
B��һ�������£���0��5mol N2��1��5m01H2�����ܱ������г�ַ�Ӧ����NH3����19��3kJ���Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)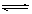 2NH3(g)��H =-38��6kJ/mol 2NH3(g)��H =-38��6kJ/mol |
| C����l01lkPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285��8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2(g)+O2(g)�T2H2O(l)��H =-571��6kJ/mol |
| D��HCI��NaOH��Ӧ���к��ȡ�H = -57��3kJ/mol����H2SO4��Ca(OH)2����Ӧ���к��ȡ�H = 2��(-57��3)kJ/mol |
��֪CO��g��+H2O��g�� H2��g��+CO2��g����H��0���������������䣬����˵������ȷ����
H2��g��+CO2��g����H��0���������������䣬����˵������ȷ����
| A������������ı��˷�Ӧ��;������Ӧ�ġ�HҲ��֮�ı� |
| B�������¶ȣ���Ӧ���ʼӿ죬��Ӧ�ų����������� |
| C���ı�ѹǿ��ƽ�ⲻ�����ƶ�����Ӧ�ų����������� |
| D������ԭ����н��У���Ӧ�ų����������� |
��б������������������ͬ�������塣
��֪����S(s����б)+O2(g)��SO2 (g) ��H1��-297.16kJ��mol-1
��S(s������)+O2(g)��SO2 (g) ��H2��-296.83kJ��mol -1
����˵����ȷ����
| A��S(s����б)��S(s������)��H3��+0.33kJ��mol -1 |
| B��������ȵ�б���ȶ� |
| C����ͬ���ʵ�����������ȵ�б�������е������� |
| D����ʽ��ʾ����lmol O2�еĹ��ۼ������յ��������γ�1mol SO2�еĹ��ۼ����ų���������297.16kJ |
�к��Ȳⶨʵ���У�ͨ���������ȼ�ֱ�Ӳ�õ�������
| A����Ӧ�������仯 | B����ϵ���¶ȱ仯 |
| C�������Ũ�ȱ仯 | D������ˮ�����ʵ��� |
���з�Ӧ����������ԭ��Ӧ���������ȷ�Ӧ���ǣ� ��
| A��ʵ�����Ʊ����� | B��Ba(OH)2��8H2O��NH4Cl�ķ�Ӧ |
| C�����ȵ�̼��ˮ�ķ�Ӧ | D����������������ĩ��Ӧ |