��Ŀ����
��п�������Ĺ�ҵ��������п�������˺�Zn�⣬������Fe��Al��Cd��SiO2�����ʣ�������п����ȡ������ZnSO4��7H2O�ͽ����ӣ�Cd����һ������ij��ԣ����������£�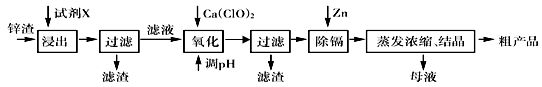
��֪��Fe3+��Al3+��Zn2+��Cd2+��Fe2+������������ȫ����ʱ��pH�ֱ�Ϊ��3.2��4.7��6.5��9.4��9.7���Իش��������⣺
��1������ʱ�õ����Լ�XΪ �� д���ܽ�����мӿ�������ʺ���߽����ʵ������ʩ��_______________________________��______________________________��
��2��д������Ca(ClO)2��Ӧ�����ӷ���ʽ ��
��3������pH���̿���ѡ�� ���ZnO����NaOH�����������̵�pH����һ�����5����Ŀ���� ��
��4��д�����˺���������п�۷�Ӧ�����ӷ���ʽ ��
��5��������Ũ������ʱ��Ҫ��ȡʵ���ʩ�ǣ� ��
��1��H2SO4��Һ �ʵ����¡���ֽ��衢�ӳ��ܽ�ʱ��ȣ������𰸺���Ҳ���֣�
��2��2H++ClO��+2Fe2+ =Cl��+2Fe3++H2O����ƽ����1�֣�
��3��ZnO ����ȥFe3+��Al3+����ֹZn2+��������
��4��Zn+Cd2+=Zn2++Cd ��5������һ������ȣ������������Ҳ���֣�
���������������1���������Ʊ�����п����ĵĹ������̣�п���к������ܵĶ����������ʣ�ѡ�����ܳ�ȥ�������裬���Ǿ������������ʣ�Ӧѡ������������ӵ�������Һ�����ܽ⡣��Ҫ�ӿ�������ʺ���߽����ʣ������ʵ������¶Ȼ������Һ���ʵ��ӳ�ʱ��ȡ�
��2���������������Һ�к�������Fe3+��Al3+��Zn2+��Cd2+��Fe2+ �����ӣ�������Щ���ӷֱ������pHֵ��Ӧ�ð�Fe2+ ����ΪFe3+ �ڳ�����ȥ�����Լ���������Ca(ClO)2 �����ķ�Ӧ��ʽΪ��2H++ClO��+2Fe2+ =Cl��+2Fe3++H2O��
��3���������������Ʊ���������п���壬�����ڵ���pHʱҪע�ⲻ���������ʣ�����Ӧ��ѡ��ZnO���ڣ�������pHֵ��5����Ŀ����ʹ���׳�����Fe3+��Al3+ �ȳ�����ȥ����ֹpH���߰����ɵ�Al(OH)3�ܽ⡢��Zn2+ ������
��4�����ϲ�������pH����Һ�л�������������Cd2+ ������Cd2+ ������pHֵ��Zn2+ ������pHֵ��������Բ����ó�������ȥ������Ҫ���뵥��Zn��Cd�û���������˷��������ӷ���ʽΪ��Zn+Cd2+=Zn2++Cd��
��5������п������Ũ��ʱ������ˮ�⣬����Ϊ�˼���ˮ��������ʣ�Ӧ�ñ�����Һһ������ȡ�
���㣺���⿼����ǻ�ѧ���������⣬���⿼�ıȽϻ������������ͽ϶ࡣ

10��ʱ���ȱ���NaHCO3��Һ����ø���Һ��pH���������ʾ�ı仯��
| �¶�/�� | 10 | 20 | 30 | ������к���ȴ��50�� |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
��ͬѧ��Ϊ������ҺpH�����ԭ����HCO
 ��ˮ��̶����ʼ�����ǿ���йط�Ӧ�����ӷ���ʽΪ__________________________����ͬѧ��Ϊ����ҺpH�����ԭ����NaHCO3���ȷֽ�������Na2CO3�����ƶ�Na2CO3��ˮ��̶�________(����ڡ���С�ڡ�)NaHCO3��ˮ��̶ȣ��÷ֽⷴӦ�Ļ�ѧ����ʽΪ______________________________��
��ˮ��̶����ʼ�����ǿ���йط�Ӧ�����ӷ���ʽΪ__________________________����ͬѧ��Ϊ����ҺpH�����ԭ����NaHCO3���ȷֽ�������Na2CO3�����ƶ�Na2CO3��ˮ��̶�________(����ڡ���С�ڡ�)NaHCO3��ˮ��̶ȣ��÷ֽⷴӦ�Ļ�ѧ����ʽΪ______________________________����ͬѧ��Ϊ���ס��ҵ��ж϶�����֣�����������̽������֤���ǵ��ж��Ƿ���ȷ��
(1)�ڼ�����е���Һ�м����������Լ�X����������������________(��ס����ҡ�)���ж���ȷ���Լ�X��________(�����)��
A��Ba(OH)2��Һ B��BaCl2��Һ
C��NaOH��Һ D������ʯ��ˮ
(2)�����Ⱥ����Һ��ȴ��10�棬����Һ��pH________(����ڡ���С�ڡ����ڡ�)8.3����________(��ס����ҡ�)���ж���ȷ��
(3)��ͬѧ�������Ϻ��֣�NaHCO3�ķֽ��¶�Ϊ150�棬������________(��ס����ҡ�)���ж��Ǵ���ģ�ԭ����________________________________________________��
�������������������ܽ�Ȳ�ͬ����˿���������һ���ʣ�������Һ��pH���ﵽ����������ӵ�Ŀ�ġ����ܽ��������������ڲ�ͬpH�µ��ܽ��(S/ mol��L��1)��ͼ��ʾ��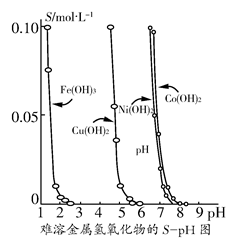
(1)pH��3ʱ��Һ��ͭԪ�ص���Ҫ������ʽ��________��
(2)��Ҫ��ȥCuCl2��Һ�е�����Fe3����Ӧ�ÿ�����Һ��pH________��
A��<1����B��4���ҡ���C��>6
(3)��Ni(NO3)2��Һ�к���������Co2�����ʣ�________(��ܡ����ܡ�)ͨ��������ҺpH�ķ�������ȥ��������__________________________��
(4)Ҫʹ������ͭ�����ܽ⣬���˼�����֮�⣬�����Լ��백ˮ����[Cu(NH3)4]2����д����Ӧ�����ӷ���ʽ��________________________��
(5)��֪һЩ��������ܶȻ��������±���
| ���� | FeS | MnS | CuS |
| Ksp | 6.3��10��18 | 2.5��10��13 | 1.3��10��35 |
| ���� | PbS | HgS | ZnS |
| Ksp | 3.4��10��28 | 6.4��10��33 | 1.6��10��24 |
Ϊ��ȥij��ҵ��ˮ�к��е�Cu2����Pb2����Hg2�����ʣ���������˹�ҵ��ˮ�м��������________��
A��NaOH������B��FeS������C��Na2S
ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ�飺
ʵ��һ�����Ʋ��궨������Һ��Ũ��
ȡ����������250 mL 0.2 mol��L��1�Ĵ�����Һ����0.2 mol��L��1�Ĵ�����Һϡ�ͳ�����Ũ�ȵ���Һ������NaOH����Һ�����������Һ��Ũ�Ƚ��б궨���ش��������⣺
(1)����250 mL 0.2 mol��L��1������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�����������________��________��
(2)Ϊ�궨ij������Һ��ȷŨ�ȣ���0.2000 mol��L��1��NaOH��Һ��20.00 mL������Һ���еζ������εζ�����NaOH��Һ��������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�����(mL) | 20.05 | 20.00 | 18.80 | 19.95 |
��ô�����Һ��ȷŨ��Ϊ________��(����С�������λ)
ʵ�����̽��Ũ�ȶԴ������̶ȵ�Ӱ��
��pH�Ʋⶨ25��ʱ��ͬŨ�ȵĴ����pH��������£�
| ����Ũ��(mol��L��1) | 0.0010 | 0.0100 | 0.0200 | 0.1000 | 0.2000 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
�ش��������⣺
(1)���ݱ������ݣ����Եó�������������ʵĽ��ۣ�����Ϊ�ó��˽��۵������ǣ�____________________��
(2)�ӱ��е����ݣ������Եó���һ���ۣ����Ŵ���Ũ�ȵļ�С������ĵ���̶Ƚ�________��(�������С�����䡱)
ʵ������̽���¶ȶԴ������̶ȵ�Ӱ��
�������һ��ʵ����ɸ�̽������������ʵ�鷽����____________��
�±��Dz�ͬ�¶���ˮ�����ӻ�������
| �¶�/�� | 25 | T1 | T2 |
| ˮ�����ӻ����� | 1��10��14 | a | 1��10��12 |
�Իش��������⣺
(1)��25<T1<T2����a__________1��10��14 (�>������<������)�������жϵ�������______________________________________��
(2)25��ʱ��ijNa2SO4��Һ��c(SO42��)��5��10��4 mol/L��ȡ����Һ1 mL��ˮϡ����10 mL����ϡ�ͺ���Һ��c(Na��)��c(OH��)��__________��
(3)T2��ʱ����pH��11�Ŀ�������ҺV1 L��pH��1��ϡ����V2 L���(���Ϻ���Һ�����Ϊԭ����Һ���֮��)�����û����Һ��pH��2����V1��V2��__________������Һ�и������ӵ�Ũ���ɴ�С��˳����____________________________��
(4)�����£���ijpHֵ��������ˮ�������c(H��)��1.0��10��a mol/L������ͬpHֵ����������ˮ�������c(H��)��1.0��10��b mol/L��(a��b����С��14������)����ôa��b֮������Ĺ�ϵʽ��________________��
��ҵ����̼���̿�Ϊ��Ҫԭ������MnO2�Ĺ����������£�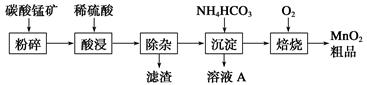
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Pb(OH)2 | Mn(OH)2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
��ش��������⣺
(1)���ǰ��̼���̿�����������__________________��
(2)��������Һ�к���Mn2����SO42��������������Fe2����Fe3����Al3����Cu2����Pb2���ȣ�����ӹ������£�
�ټ���MnO2��Fe2�������������ӷ�Ӧ����ʽΪ__________________________��
�ڼ���CaO����Һ��pH����5.2��6.0������ҪĿ����
_____________________________________________________________��
�ۼ���BaS����ȥCu2����Pb2�����ټ���NaF��Һ����ȥ______________________��
(3)����ҺA�л��յ���Ҫ������________________�������ʳ��������ʡ�
(4)MnO2��Ʒ�к�������Mn3O4��������ϡ���ᴦ��������ת��ΪMnSO4��MnO2��Ȼ��������������Mn2��ת��ΪMnO2���Ƶ�����MnO2��д��Mn3O4��ϡ���ᷴӦ�Ļ�ѧ����ʽ��______________________________��
 Sr2��(aq)��SO42��(aq)��Ksp��2.5��10��7
Sr2��(aq)��SO42��(aq)��Ksp��2.5��10��7