��Ŀ����
����Ŀ���ñ�ǩ��ʾŨ��������250mL 0.4mol/L��ϡ���ᣬ�������й�ʵ�飮 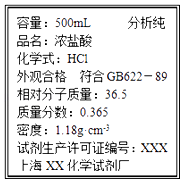
��ش�
��1����Ҫ��ȡŨ����mL��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ����������⣬�������õ����������� ��
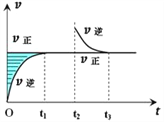
��3����ʵ���������������������Һ��Ũ�Ȼᣨ�ƫ�ߡ�����ƫ�͡��������䡱�� A������ʱ���ӿ̶�����
B������ƿ�ڱڸ���ˮ���δ���ﴦ����
C���ܽ��û����ȴ����ж��� ��
��4���������Ƶ�ϡ���ᵹ��5.92g Na2CO3��NaHCO3�Ĺ��������У�ǡ����ȫ��Ӧ����ԭ�������Na2CO3����������Ϊ ��
���𰸡�
��1��8.5
��2����ͷ�ιܣ�250mL����ƿ
��3��ƫ�ͣ����䣻ƫ��
��4��71.6%
���������⣺��1��Ũ��������ʵ���Ũ��C= ![]() =11.8mol/L������ҪŨ�������ΪV��������Һϡ���������ʵ����ʵ�������ã�V��11.8mol/L=250mL��0.4mol/L�����V=8.5mL�� ���Դ��ǣ�8.5����2������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݵȣ��õ�����������Ͳ����ͷ�ιܡ��ձ�����������250mL����ƿ���������õ�����������ͷ�ι� 250mL����ƿ��
=11.8mol/L������ҪŨ�������ΪV��������Һϡ���������ʵ����ʵ�������ã�V��11.8mol/L=250mL��0.4mol/L�����V=8.5mL�� ���Դ��ǣ�8.5����2������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݵȣ��õ�����������Ͳ����ͷ�ιܡ��ձ�����������250mL����ƿ���������õ�����������ͷ�ι� 250mL����ƿ��
���Դ��ǣ���ͷ�ιܣ�250mL����ƿ�� ��3��A������ʱ���ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ͣ�
B������ƿ�ڱڸ���ˮ���δ���ﴦ���������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣻
���Դ��ǣ����䣻
C���ܽ��û����ȴ����ж��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ�
���Դ��ǣ�ƫ�ߣ���4������Na2CO3��NaHCO3������ֱ�ΪXmol��Ymol����
106X+84Y=5.92��
��250mL 0.4mol/L��ϡ���ᣬ���Ȼ����������Ϊ��0.4mol/L��0.25L=0.1mol��
���ݷ���ʽ��Na2CO3+2HCl=2NaCl+H2O+CO2�� NaHCO3 +HCl=NaCl+H2O+CO2����̼���ƣ�̼��������ȫ��Ӧ�����Ȼ��ƣ�������ԭ�Ӹ����غ㣬��֪�����Ȼ������ʵ���Ϊ0.1mol��
��2X+Y=0.1��
106X+84Y=5.92��
2X+Y=0.1��
��ã�Y=0.02��X=0.04��
��̼���Ƶ����������� ![]() ��1005=71.6%
��1005=71.6%
���Դ��ǣ�71.6%��