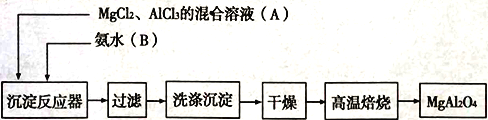
��Ŀ����
����Ŀ��ͼΪŨ������ͭƬ��Ӧ��װ�ã���ش�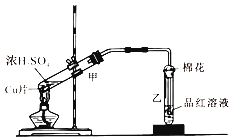
��1��Ũ������ͭƬ��Ӧ�Ļ�ѧ����ʽΪ
��2����Ӧһ��ʱ����Թ�����Ʒ����Һ��������
��3�������йظ�ʵ���˵���У�����ȷ���ǡ� ����
A���÷�Ӧ��Ũ����ֻ��������
B���Թ����к��м�Һ�����������������չ�����SO2 �� ��ֹ������Ⱦ
C����Ӧһ��ʱ����Թܼ��е���Һ��������ʢ��ˮ���ձ�����Һ����ɫ
D����0.04mol���ʵ�Ũ������������ͭƬ��Ӧ�����ռ���448mL��SO2����״����
���𰸡�
��1��Cu+2H2SO4![]() CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
��2��Ʒ����Һ��ɫ
��3��AD
����������1��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Cu+2H2SO4![]() CuSO4+SO2��+2H2O���ʴ�Ϊ��Cu+2H2SO4
CuSO4+SO2��+2H2O���ʴ�Ϊ��Cu+2H2SO4![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
��2�����������ܺ���ɫ����������ɫ���ʣ�����������ʹƷ����Һ��ɫ�����Զ����������Ư���ԣ������ɵ���ɫ���ʲ��ȶ�������ʱ��ָ�ԭ������ɫ��
�ʴ�Ϊ��Ʒ����Һ��ɫ��
��3����3��A���÷�Ӧ��Ũ����ֻ�������Ժ������ԣ���A����
B���Թ����к��м�Һ��������ֹ��������ɢʧ�������У���ɴ�����Ⱦ�������������չ�����SO2 �� ��ֹ������Ⱦ����B��ȷ��
C����Ӧһ��ʱ����Թܼ��е���Һ��������ʢ��ˮ���ձ�������ͭ���ӵ���Һ����Һ����ɫ����C��ȷ��
D����0.04mol���ʵ�Ũ������������ͭƬ��Ӧ�����ŷ�Ӧ�Ľ���Ũ�ȼ�С��ϡ������ͭ����Ӧ�������ռ���SO2������ڱ�״��С��448mL����D����ѡ��AD��
��1��ͭ��Ũ���ᷴӦ��������ͭ�����������ˮ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��2������������ʹƷ����Һ��ɫ�������������Ư���Բ��ȶ�������ʱ��ָ�ԭ������ɫ��
��3��A���÷�Ӧ��Ũ����ֻ�������Ժ������ԣ�
B���Թ����к��м�Һ��������ֹ��������ɢʧ�������У���ɴ�����Ⱦ��
C����Ӧһ��ʱ����Թܼ��е���Һ��������ʢ��ˮ���ձ�������ͭ���ӵ���Һ����Һ����ɫ��
D����0.04mol���ʵ�Ũ������������ͭƬ��Ӧ�����ŷ�Ӧ�Ľ���Ũ�ȼ�С��ϡ������ͭ����Ӧ��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�����Ŀ��ֻ�������м��Ҷ�Ӧ�������������������ʵ�������
�� | �� | |
�� | �������� | �����ӵ����� |
�� | ��״��������Ħ����� | ��״����������� |
�� | ������� | �����ܶ� |
�� | ��Һ�������� | ��Һ��� |
�� | �DZ�״�������ʵ����� | ���ʵ�Ħ������ |
A. �ڢۢ� B. �ۢܢ� C. �ۢ� D. ��