��Ŀ����
��NAΪ�����ӵ�������ֵ�����������в���ȷ����
[ ]
��12.4g�������к��е�p-p������0.6NA
�ڵ�⾫��ͭʱת����NA�����ӣ������ܽ�32gͭ
��100g��98%��ŨH2SO4����������ԭ����Ϊ4NA
��2mol SO2��1mol O2�����V2O5���ڵ����������ܱ������м��ȷ�Ӧ�����������ʷ���������2NA
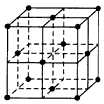
��2.9g 2CaSO4��H2O���еĽᾧˮ������Ϊ0.02NA
��C60�����ṹ��ͼ��ʾ��720gC60���庬NA������
�ߺ�1mol FeCl3������Һ���뵽��ˮ�γ������������壬��������ĿԼΪNA
A.�ڢۢݢޢ�
B.�٢ۢܢݢ�
C.�٢ڢۢܢ�
D.�ۢܢݢޢ�
�ڵ�⾫��ͭʱת����NA�����ӣ������ܽ�32gͭ
��100g��98%��ŨH2SO4����������ԭ����Ϊ4NA
��2mol SO2��1mol O2�����V2O5���ڵ����������ܱ������м��ȷ�Ӧ�����������ʷ���������2NA
��2.9g 2CaSO4��H2O���еĽᾧˮ������Ϊ0.02NA
��C60�����ṹ��ͼ��ʾ��720gC60���庬NA������
�ߺ�1mol FeCl3������Һ���뵽��ˮ�γ������������壬��������ĿԼΪNA
A.�ڢۢݢޢ�
B.�٢ۢܢݢ�
C.�٢ڢۢܢ�
D.�ۢܢݢޢ�
A

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��NAΪ�����ӵ�����ֵ������˵������ȷ����
| A��1 mol O2������Ʒ�Ӧ��O2��һ���õ�4NA������ |
| B����״���£�a L CH4��a L C6H14��������������Ϊa NA/22.4 |
| C�����³�ѹ�£�1 mol�����������Ľ���þ��Ӧ��ת��2NA������ |
| D����20 �桢1.01��105 Paʱ��2.8 g C2H4��CO�Ļ�����庬�������������0.1 NA |