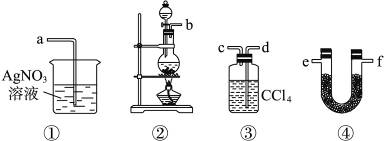
��Ŀ����
���Ʒ��Ƽ��ԭ���DZ���ʳ��ˮ�����ڴ����к�����ɳ��Ca2+��Mg2+��Fe3+��SO42�D���ʣ������ϵ��Ҫ����˱��뾭�����ơ�ijУʵ��С�龫�ƴ���ˮ��ʵ��������£�

��ش��������⣺
��1������a��������____________������������________________________________��
��2���ڢ��У���������Լ������������ִ����ij��������Լ���Ϊ________��Һ��
��3���ڵڢ����У������Լ���ֱ����Һ�����Ա仯ʱ��д���˹��̵Ļ�ѧ����ʽ________________��

��ش��������⣺
��1������a��������____________������������________________________________��
��2���ڢ��У���������Լ������������ִ����ij��������Լ���Ϊ________��Һ��
��3���ڵڢ����У������Լ���ֱ����Һ�����Ա仯ʱ��д���˹��̵Ļ�ѧ����ʽ________________��
��1�����ˡ� ��������©�����ձ�
��2��NaOH
��3��HCl+NaOH==NaCl+H2O�� Na2CO3+2HCl==2NaCl+H2O+CO2��
��2��NaOH
��3��HCl+NaOH==NaCl+H2O�� Na2CO3+2HCl==2NaCl+H2O+CO2��
˼·�㲦�����⿼��������ᴿ�е�������⡣�������뷽����ѡ�����������������Լ���ѡ��ȵȡ�
������
��1������ɳ�Ȳ�������������Һ����IJ����ǹ��ˣ����˲��������貣�������Dz�������©�����ձ�����2�������Լ����������ֳ����������������ӱ�Ȼ��Mg(OH)2��Fe(OH)3�����Լ�ΪNaOH����3��Һ��F���й�����NaOH��Na2CO3��Ӧ��������ɽ����ȥ��
������
��1������ɳ�Ȳ�������������Һ����IJ����ǹ��ˣ����˲��������貣�������Dz�������©�����ձ�����2�������Լ����������ֳ����������������ӱ�Ȼ��Mg(OH)2��Fe(OH)3�����Լ�ΪNaOH����3��Һ��F���й�����NaOH��Na2CO3��Ӧ��������ɽ����ȥ��

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�
�����Ŀ