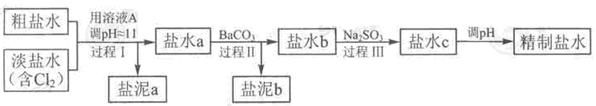
��Ŀ����
����ͼ��ʾ�����£�U�ι���ʢ��100mL��ij����Һ��
�밴Ҫ��ش��������⡣
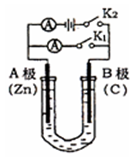
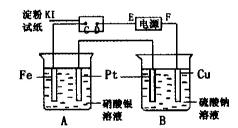
��1������ʢ��ҺΪCuSO4��Һ����K2���ϲ�K1����
�� AΪ ����B���ĵ缫��ӦʽΪ ��
�ڷ�Ӧ�����У���Һ��SO42����OH�������� �����A��B��
�ƶ���
��2������ʢ��ҺΪ���з�̪��NaCl��Һ����K1���ϲ�K2����
��A�缫�ɹ۲쵽�������� ��
�ڵ������ܷ�Ӧ�Ļ�ѧ����ʽ�� ��
�۷�Ӧһ��ʱ����K2 ,��������Һ������仯��������ܽ⣬B��������������������ɱ�״����Ϊ11.2mL������Һ��ֻ�ϣ���Һ��pHΪ ��
�������ĵ������Һ�м����ͨ�� �����Լ����ƣ�����ʹ��Һ��ԭ��
�밴Ҫ��ش��������⡣

��1������ʢ��ҺΪCuSO4��Һ����K2���ϲ�K1����
�� AΪ ����B���ĵ缫��ӦʽΪ ��
�ڷ�Ӧ�����У���Һ��SO42����OH�������� �����A��B��
�ƶ���
��2������ʢ��ҺΪ���з�̪��NaCl��Һ����K1���ϲ�K2����
��A�缫�ɹ۲쵽�������� ��
�ڵ������ܷ�Ӧ�Ļ�ѧ����ʽ�� ��
�۷�Ӧһ��ʱ����K2 ,��������Һ������仯��������ܽ⣬B��������������������ɱ�״����Ϊ11.2mL������Һ��ֻ�ϣ���Һ��pHΪ ��
�������ĵ������Һ�м����ͨ�� �����Լ����ƣ�����ʹ��Һ��ԭ��
��1���ٸ���Cu2++2e-=Cu ��A
��2���ٲ�������,�缫������Һ��졣��2NaCl+2H2O 2NaOH+H2��+Cl2������ 12
2NaOH+H2��+Cl2������ 12
���Ȼ���
��2���ٲ�������,�缫������Һ��졣��2NaCl+2H2O
 2NaOH+H2��+Cl2������ 12
2NaOH+H2��+Cl2������ 12 ���Ȼ���
��1���ٸ���Cu2++2e-=Cu ��A
��������K2���ϲ�K1���γ�ԭ��أ�A��п���ǻ��ý�������������BΪ�������õ��ӣ��缫��ӦΪCu2++2e-=Cu���ڸ������������Һ��SO42����OH��������A���ƶ���
��2���ٲ�������,�缫������Һ��졣��2NaCl+2H2O
 2NaOH+H2��+Cl2������ 12
2NaOH+H2��+Cl2������ 12 ���Ȼ���
��������2����K1���ϲ�K2���γɵ��أ�AΪ������2H2O��2e-=H2��2OH�D ,�ɹ۲쵽�������Ǣٲ�������,�缫������Һ��졣�ڵ������ܷ�Ӧ�Ļ�ѧ����ʽ��2NaCl+2H2O
 2NaOH+H2��+Cl2������B������Cl2��11.2mL/22400ml��mol-1=0.0005mol,�ɷ���ʽ������0.001molNaOH ,c(NaOH)=0.001mol/0.1L=0.01mol / L,pH="14+lg0.01" ="12"
2NaOH+H2��+Cl2������B������Cl2��11.2mL/22400ml��mol-1=0.0005mol,�ɷ���ʽ������0.001molNaOH ,c(NaOH)=0.001mol/0.1L=0.01mol / L,pH="14+lg0.01" ="12" ���ɷ���ʽ��֪�����Ȼ��⣬��ʹ��Һ��ԭ��

��ϰ��ϵ�д�
�����Ŀ

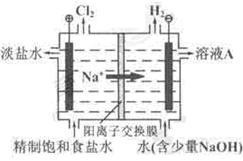
 _______
_______  ______��
______��