��Ŀ����
��12�֣��ȼҵ�е�ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ����ͼ��ʾ��
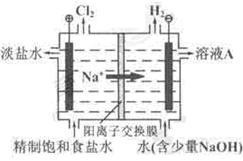
��1����ҺA�������� ��
��2����ⱥ��ʳ��ˮ�����ӷ���ʽ�� ��
��3�����ʱ�����������������Һ��pH��2��3���û�ѧƽ���ƶ�ԭ��������������� ��
��4��������õ���ˮ�辫�ơ�ȥ����Ӱ���Ca2����Mg2����NH4����SO42��[c(SO42��)��c(Ca2��)]��
�����������£�����ˮ����ҺA���Ե��أ���
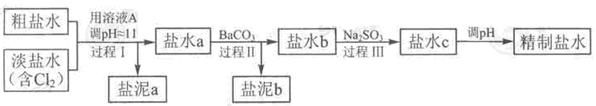
������a����ɳ�⣬�����е������� ��
�ڹ��̢��н�NH4+ת��ΪN2�����ӷ���ʽ��
��BaSO4���ܽ�ȱ�BaCO3��С�����̢��г�ȥ��������
�ܾ����̢�����Ҫ����ˮ��c ��ʣ��Na2SO3�ĺ���С��5mg /L,����ˮb��NaClO�ĺ�����7.45 mg /L ������10m3��ˮb ����������10% Na2SO3��Һ kg����Һ����仯���Բ��ƣ���

��1����ҺA�������� ��
��2����ⱥ��ʳ��ˮ�����ӷ���ʽ�� ��
��3�����ʱ�����������������Һ��pH��2��3���û�ѧƽ���ƶ�ԭ��������������� ��
��4��������õ���ˮ�辫�ơ�ȥ����Ӱ���Ca2����Mg2����NH4����SO42��[c(SO42��)��c(Ca2��)]��
�����������£�����ˮ����ҺA���Ե��أ���

������a����ɳ�⣬�����е������� ��
�ڹ��̢��н�NH4+ת��ΪN2�����ӷ���ʽ��
��BaSO4���ܽ�ȱ�BaCO3��С�����̢��г�ȥ��������
�ܾ����̢�����Ҫ����ˮ��c ��ʣ��Na2SO3�ĺ���С��5mg /L,����ˮb��NaClO�ĺ�����7.45 mg /L ������10m3��ˮb ����������10% Na2SO3��Һ kg����Һ����仯���Բ��ƣ���
��12�֣�����1���͢�ÿ��1���⣬����ÿ��2�֣�
��1��NaOH��1�֣� ��2��2Cl����2H2O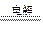 2OH����H2����Cl2��
2OH����H2����Cl2��
��3��������ˮ��Ӧ��Cl2��H2O HCl��HClO������HCl��Ũ�ȿ�ʹƽ�������ƶ�������������ˮ�е��ܽ⣬�����������������
HCl��HClO������HCl��Ũ�ȿ�ʹƽ�������ƶ�������������ˮ�е��ܽ⣬�����������������
��4����Mg(OH)2 ��1�֣� ��2NH4����3Cl2��8OH��=8H2O��6Cl����N2��
��SO42����Ca2�� ��1.76�� NaClO����ǿ�����ԣ��ɽ�Na2SO3������Na2SO4������ʽΪNa2SO3+NaClO��Na2SO4+NaCl��10m3��ˮb�к�NaClO�����ʵ���Ϊ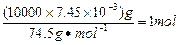 ���ɷ���ʽ��֪����Na2SO3������Ϊ1mol��126g/mol��126g��������Ҫ10% Na2SO3��Һ������ΪX������
���ɷ���ʽ��֪����Na2SO3������Ϊ1mol��126g/mol��126g��������Ҫ10% Na2SO3��Һ������ΪX������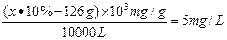 �����x��1760g������������10% Na2SO3��Һ1.76kg����
�����x��1760g������������10% Na2SO3��Һ1.76kg����
��1��NaOH��1�֣� ��2��2Cl����2H2O
 2OH����H2����Cl2��
2OH����H2����Cl2����3��������ˮ��Ӧ��Cl2��H2O
 HCl��HClO������HCl��Ũ�ȿ�ʹƽ�������ƶ�������������ˮ�е��ܽ⣬�����������������
HCl��HClO������HCl��Ũ�ȿ�ʹƽ�������ƶ�������������ˮ�е��ܽ⣬�������������������4����Mg(OH)2 ��1�֣� ��2NH4����3Cl2��8OH��=8H2O��6Cl����N2��
��SO42����Ca2�� ��1.76�� NaClO����ǿ�����ԣ��ɽ�Na2SO3������Na2SO4������ʽΪNa2SO3+NaClO��Na2SO4+NaCl��10m3��ˮb�к�NaClO�����ʵ���Ϊ
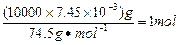 ���ɷ���ʽ��֪����Na2SO3������Ϊ1mol��126g/mol��126g��������Ҫ10% Na2SO3��Һ������ΪX������
���ɷ���ʽ��֪����Na2SO3������Ϊ1mol��126g/mol��126g��������Ҫ10% Na2SO3��Һ������ΪX������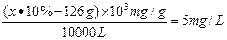 �����x��1760g������������10% Na2SO3��Һ1.76kg����
�����x��1760g������������10% Na2SO3��Һ1.76kg������

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
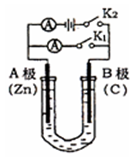
 ������˵����ȷ����
������˵����ȷ����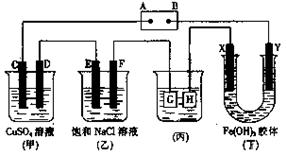
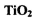 �ڹ�ҵ�������ճ�����������Ҫ��;�� I.��ҵ�����ѿ�ʯ(
�ڹ�ҵ�������ճ�����������Ҫ��;�� I.��ҵ�����ѿ�ʯ( ,��
,��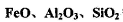 �����ʣ�����������Ӧ�Ƶã�
�����ʣ�����������Ӧ�Ƶã�
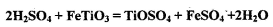
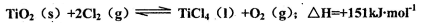
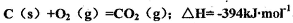
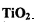 ��Cl2��C��Ӧ��ȡTiO4���Ȼ�ѧ����ʽ��____________
��Cl2��C��Ӧ��ȡTiO4���Ȼ�ѧ����ʽ��____________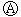 Ϊ
Ϊ ֱ����Դ��
ֱ����Դ��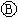 Ϊ�������Ȼ�����Һ�ͷ�̪��Һ����ֽ��
Ϊ�������Ȼ�����Һ�ͷ�̪��Һ����ֽ��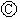 Ϊ��Ʋۣ���ͨ��·����
Ϊ��Ʋۣ���ͨ��·���� ��ʹc��d�����·��
��ʹc��d�����·��