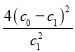题目内容
【题目】〔化学——选修3:物质结构与性质〕举世瞩目的中国探月工程三期再入返回试验器于2014年10月24日凌晨成功发射,为实现月球采样和首次地月往返踏出了成功的第一步。探月工程三期的主要目标是实现无人自动采样返回,突破月面采样、月面上升、月球轨道交会对接等核心关键技术。已知所用火箭推进剂为肼(N2H4)和过氧化氢(H2O2),火箭箭体一般采用钛合金材料。
请回答下列问题:
(1)N2H4、H2O2分子中电负性最大的元素在周期表中的位置为 ,第一电离能最大的元素为 。
(2)钛的原子序数为22,其基态电子排布式示意图为 。
(3)1 mol N2H4分子中含有的键数目为 。
(4)H2O2分子结构如图1,其中心原子杂化轨道为 ,估计它难溶于CS2,简要说明原因 。
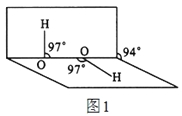
(5)氮化硼其晶胞如图2所示,则处于晶胞顶点上的原子的配位数为 ,若立方氮化硼的密度为g·cm-3,阿伏加德罗常数为NA,则两个最近N原子间的距离为________cm。
【答案】(1)第二周期ⅥA族 N
(2)![]()
(3)3.01×1024
(4)sp3 H2O2为极性分子,CS2为非极性溶剂,所以H2O2不能溶解在CS2中
(5)4
【解析】
试题分析:(1)N2H4、H2O2共有H、O、N三种元素,根据非金属性越强,其电负性越大,可知O元素为电负性最大的元素,O元素位于周期表中第二周第ⅥA族。由于N原子2p3上的电子处于半充满状态,很难失去1个电子,所以第一电离能最大。 (2)Ti的原子序数为22,Ti原子核外有22个电子,根据核外电子排布规律和相关规则,其基态电子排布示意图为![]() 。 (3)N2H4中的N原子满足8电子结构,H原子满足2电子结构,N2H4的分子结构简式为H2N―NH2,可见1molN2H4中含有5mol键,其数目=5×6.02×1023个=3.01×1024个。 (4)H2O2中中心原子为O原子,O原子成2个σ键,有2对孤对电子,原子杂化轨道数=键数+孤对电子对数=2+2=4,故H2O2分子的中心原子采用sp3杂化。由H2O2的结构可知,H2O2为极性分子,而CS2为非极性溶剂,根据“相似相容原理”,H2O2不能溶解在CS2中。(5)晶胞顶点上的N原子,距其最近有4个B原子(晶胞的上、下、左、右各1个),其配位数为4。根据“均摊法”可知,1个氮化硼晶胞中含有N元素数=8×1/8+6×1/2=4,B原子全部位于晶胞内,共4个B原子,一个晶胞的质量为
。 (3)N2H4中的N原子满足8电子结构,H原子满足2电子结构,N2H4的分子结构简式为H2N―NH2,可见1molN2H4中含有5mol键,其数目=5×6.02×1023个=3.01×1024个。 (4)H2O2中中心原子为O原子,O原子成2个σ键,有2对孤对电子,原子杂化轨道数=键数+孤对电子对数=2+2=4,故H2O2分子的中心原子采用sp3杂化。由H2O2的结构可知,H2O2为极性分子,而CS2为非极性溶剂,根据“相似相容原理”,H2O2不能溶解在CS2中。(5)晶胞顶点上的N原子,距其最近有4个B原子(晶胞的上、下、左、右各1个),其配位数为4。根据“均摊法”可知,1个氮化硼晶胞中含有N元素数=8×1/8+6×1/2=4,B原子全部位于晶胞内,共4个B原子,一个晶胞的质量为![]() g,设该立方氮化硼晶胞的边长为acm,则1个立方氮化硼晶胞的体积是a3,因此立方氮化硼的密度=
g,设该立方氮化硼晶胞的边长为acm,则1个立方氮化硼晶胞的体积是a3,因此立方氮化硼的密度=![]() g·cm-3,解得a=,两个最近N原子间的距离是此晶胞的边长的,即为。
g·cm-3,解得a=,两个最近N原子间的距离是此晶胞的边长的,即为。

 同步轻松练习系列答案
同步轻松练习系列答案 课课通课程标准思维方法与能力训练系列答案
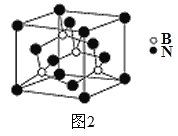
课课通课程标准思维方法与能力训练系列答案【题目】尿素[CO(NH2)2]是含氮量最高的氮肥。
(1) 已知工业上合成尿素的反应分为如下两步:
第1步:2NH3(I)+CO2(g) ![]() H2NCOONH4(I) H1=-330.0kJ·mol-1
H2NCOONH4(I) H1=-330.0kJ·mol-1
第2步:H2NCOONH4(I) ![]() H2O(I)+CO(NH2)2(I) H2=+226.3 kJ·mol-1
H2O(I)+CO(NH2)2(I) H2=+226.3 kJ·mol-1
则下列各图能正确表示尿素合成过程中能量变化曲线的是___________(填字母编号)。
(2)一定条件下工业合成尿素的总反应:CO2(g)+2HH3(g) ![]() CO(NH2)2(g)+H2O(g),t℃时,向容积恒定为2L的密闭容器中加入0.20molCO2和0.80molNH3,反应中CO2的物质的量随时间变化如下表所示:
CO(NH2)2(g)+H2O(g),t℃时,向容积恒定为2L的密闭容器中加入0.20molCO2和0.80molNH3,反应中CO2的物质的量随时间变化如下表所示:
时间/min | 0 | 40 | 70 | 80 | 100 |
n(CO2)/mol | 0.20 | 0.12 | 0.10 | 0.10 | 0.10 |
①前40min内v(NH3)=_________,此温度下该反应的平衡常数为___________。
②30min时v正(CO2)________80min时v逆(H2O)(选填“>”、“=”、“<”)。
③在100min时,保持其它条件不变,再向容器中充入0.10molCO2和0.4molNH3,重新建立平衡后CO2的转化率与原平衡相比将________(填“增大”、“不变”、“减小”)
④氨碳比[n(NH3)/n(CO2)]对合成尿素有影响,恒温恒容条件下将总物质的量为3mol的NH3和CO2的混合气体按不同氨碳比进行反应,结果如图1所示。a、b线分别表示CO2或NH3的转化率变化,c线表示平和体系中尿素的体积分数变化。[n(NH3)/n(CO2)]=______时,尿素产量最大,经计算图中y=_______(精确到0.01)。
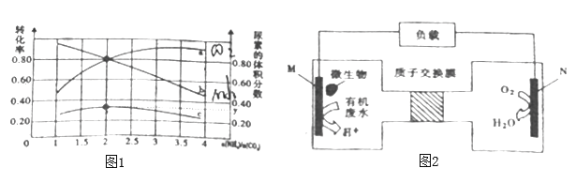
(3)工业生成中为提高尿素的产率可采取的措施有___________________。
(4)近期科学家发现微生物可将生产废水中的尿素直接转化为对环境友好的两种物质,其工作原理如图2所示。回答下列问题:
①N极为_________极(填“正”、“负”),M电极反应式________________。
②N极消耗标准状况下33.6L气体时,M极理论上处理的尿素的质量为__________g。
【题目】实验室制取乙酸丁酯的实验装置有以下甲、乙两种装置可供选用。

制备乙酸丁酯所涉及的有关物质的物理性质见下表:
乙酸 | 1-丁醇 | 乙酸丁酯 | |
熔点(℃) | 16.6 | -89.5 | -73.5 |
沸点(℃) | 117.9 | 117 | 126.3 |
密度(g/cm3) | 1.05 | 0.81 | 0.88 |
水溶性 | 互溶 | 可溶(9g/100g水) | 微溶 |
(1)仪器M的名称为_________。
(2)制取乙酸丁酯选用乙装置而不选用甲装置的理由是__________。
(3)从制备乙酸丁酯所得的混合物中分离、提纯乙酸丁酯时,需要经过多步操作,下列图示的操作中,肯定需要的化学操作是______(选填答案编号)。

(4)有机物的分离操作中,经常需要使用分液漏斗等仪器。使用分液漏斗前必须_____(填写操作)。某同学在进行分液操作时,若发现液体流不下来,其可能原因除分液漏斗活塞堵塞外,还可能_________(写出一点)。