��Ŀ����
��2010?��������ҪԪ��W��X��Y��Z��ԭ������һ������W��ԭ�������������Ǵ�����������3����X��Y��Z������ͬ�����ڣ����ǵ�ԭ������֮����Wԭ��������5��������Ԫ��W��X��Y��Z��ɵ����п��ܵĶ���ֻ������У���Ԫ��W��Y�γɵĻ�����M���۵���ߣ���ش��������⣺
��1��WԪ��ԭ�ӵ�L������Ų�ʽΪ
��2��X������ˮ������Ҫ��Ӧ�Ļ�ѧ����ʽΪ
��3��������M�Ļ�ѧʽΪ
��4��X��Y��Z���γ���������ṹ�Ļ�����侧����Xռ������������ģ�Yλ�ڶ��ǣ�Z��������λ�ã���þ�������ΪX��Y��Z=
��5������Ԫ��Z���ε���ɫ��ӦΪ
��1��WԪ��ԭ�ӵ�L������Ų�ʽΪ
2s22p4
2s22p4
��W3���ӵĿռ乹��ΪV��
V��
����2��X������ˮ������Ҫ��Ӧ�Ļ�ѧ����ʽΪ
2F2+2H2O=4HF+O2
2F2+2H2O=4HF+O2
����3��������M�Ļ�ѧʽΪ
MgO
MgO
���侧��ṹ��NaCl��ͬ�����۵����NaCl��M�۵�ϸߵ�ԭ�������ӵĵ�ɶࡢ���Ӱ뾶��С�������ܴ�
���ӵĵ�ɶࡢ���Ӱ뾶��С�������ܴ�
����һ�����Ļ�����ZX������M�Ͽ��Ƶ�ZX/M���������ڴ�̼����������¹��������ϳ�̼����¹�������̼������������У�̼ԭ�Ӳ��õ��ӻ���ʽ��sp2��sp3
sp2��sp3
��O-C-O�ļ���ԼΪ120��
120��
����4��X��Y��Z���γ���������ṹ�Ļ�����侧����Xռ������������ģ�Yλ�ڶ��ǣ�Z��������λ�ã���þ�������ΪX��Y��Z=
3��1��1
3��1��1
����5������Ԫ��Z���ε���ɫ��ӦΪ
��
��
ɫ����������ζ����Է�����ɫ��Ӧ����ԭ��������̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
������������Ԫ��W��X��Y��Z��ԭ��������������W��ԭ�������������Ǵ�����������3������W��2�����Ӳ㣬����������Ϊ6����WΪ��Ԫ�أ�X��Y��Z������ͬ�����ڣ�������Ԫ��W��X��Y��Z��ԭ��������������X������Ϊ��������Ԫ�أ���Ϊ�������ڣ�X��Y��Z��ԭ������֮�ʹ���Wԭ��������5�������Կ��Զ϶�XҲ�ڵڶ����ڣ���ԭ����������Ԫ�ش�XΪFԪ�أ���Y��Z��ԭ������֮��Ϊ8��5-9=31����Y���ڵ������ڣ�Z���ڵ������ڣ�Z��ԭ����������18����YΪNaԪ�أ���ZΪCaԪ�أ���YΪMgԪ�أ���ZΪKԪ�أ�X��ԭ�������������������⣬����Ԫ��W��Y�γɵĻ�����M���۵���ߣ���YΪMgԪ�أ�ZΪKԪ�أ�
����⣺����Ԫ��W��X��Y��Z��ԭ��������������W��ԭ�������������Ǵ�����������3������W��2�����Ӳ㣬����������Ϊ6����WΪ��Ԫ�أ�X��Y��Z������ͬ�����ڣ�������Ԫ��W��X��Y��Z��ԭ��������������X������Ϊ��������Ԫ�أ���Ϊ�������ڣ�X��Y��Z��ԭ������֮�ʹ���Wԭ��������5�������Կ��Զ϶�XҲ�ڵڶ����ڣ���ԭ����������Ԫ�ش�XΪFԪ�أ���Y��Z��ԭ������֮��Ϊ8��5-9=31����Y���ڵ������ڣ�Z���ڵ������ڣ�Z��ԭ����������18����YΪNaԪ�أ���ZΪCaԪ�أ���YΪMgԪ�أ���ZΪKԪ�أ�X��ԭ�������������������⣬����Ԫ��W��Y�γɵĻ�����M���۵���ߣ���YΪMgԪ�أ�ZΪKԪ�أ�
��1��WΪ��Ԫ�أ�Oԭ�ӵ�L������Ų�ʽΪ2s22p4��O3���ӽṹ��ͼ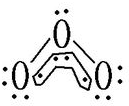 ������Oԭ�ӳ�2���Ҽ���1�������34������1�Թ¶Ե��ӣ��ӻ�������ڳɦҼ������¶Ե��Ӷԣ����ӻ������Ϊ2+1=3����������Oԭ�Ӻ���1�Թ¶Ե��ӣ���O3�ռ乹��ΪV�ͣ�
������Oԭ�ӳ�2���Ҽ���1�������34������1�Թ¶Ե��ӣ��ӻ�������ڳɦҼ������¶Ե��Ӷԣ����ӻ������Ϊ2+1=3����������Oԭ�Ӻ���1�Թ¶Ե��ӣ���O3�ռ乹��ΪV�ͣ�
�ʴ�Ϊ��2s22p4��V�ͣ�
��2��������ˮ��Ӧ����HF����������Ӧ����ʽΪ2F2+2H2O=4HF+O2���ʴ�Ϊ��2F2+2H2O=4HF+O2��
��3��������������֪��MΪMgO���侧��ṹ��NaCl��ͬ�����۵����NaCl������MgO���������ӵĵ�ɶࡢ���Ӱ뾶��С�������ܴ�MgO�۵�ϸߣ�
��̼�������������-OCH3��Cԭ��4����������ȡsp3�ӻ���������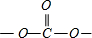 �У�Cԭ�ӳ�2��C-O���������ڦҼ���1��C=O˫����˫�����������㣬������Cԭ�ӵ��ӻ������Ϊ3����ȡsp2�ӻ���Ϊƽ���������Σ�����Ϊ120�㣬��O-C-O�ļ���ԼΪ 120�㣬
�У�Cԭ�ӳ�2��C-O���������ڦҼ���1��C=O˫����˫�����������㣬������Cԭ�ӵ��ӻ������Ϊ3����ȡsp2�ӻ���Ϊƽ���������Σ�����Ϊ120�㣬��O-C-O�ļ���ԼΪ 120�㣬
�ʴ�Ϊ��MgO�����ӵĵ�ɶࡢ���Ӱ뾶��С�������ܴ�sp2��sp3��120�㣻
��4��F��Mg��K�γ���������ṹ�Ļ����������Fռ������������ģ�������Fԭ����ĿΪ12��
=3��Mgλ�ڶ��ǣ�������Mgԭ����ĿΪ8��
=1��K��������λ�ã������к���1��Kԭ�ӣ���þ�������ΪF��Mg��K=3��1��1���ʴ�Ϊ��3��1��1��
��5������Ԫ��K���ε���ɫ��ӦΪ��ɫ����������ζ����Է�����ɫ��Ӧ����ԭ����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�������
�ʴ�Ϊ���ϣ�����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�������
��1��WΪ��Ԫ�أ�Oԭ�ӵ�L������Ų�ʽΪ2s22p4��O3���ӽṹ��ͼ
 ������Oԭ�ӳ�2���Ҽ���1�������34������1�Թ¶Ե��ӣ��ӻ�������ڳɦҼ������¶Ե��Ӷԣ����ӻ������Ϊ2+1=3����������Oԭ�Ӻ���1�Թ¶Ե��ӣ���O3�ռ乹��ΪV�ͣ�
������Oԭ�ӳ�2���Ҽ���1�������34������1�Թ¶Ե��ӣ��ӻ�������ڳɦҼ������¶Ե��Ӷԣ����ӻ������Ϊ2+1=3����������Oԭ�Ӻ���1�Թ¶Ե��ӣ���O3�ռ乹��ΪV�ͣ��ʴ�Ϊ��2s22p4��V�ͣ�
��2��������ˮ��Ӧ����HF����������Ӧ����ʽΪ2F2+2H2O=4HF+O2���ʴ�Ϊ��2F2+2H2O=4HF+O2��
��3��������������֪��MΪMgO���侧��ṹ��NaCl��ͬ�����۵����NaCl������MgO���������ӵĵ�ɶࡢ���Ӱ뾶��С�������ܴ�MgO�۵�ϸߣ�
��̼�������������-OCH3��Cԭ��4����������ȡsp3�ӻ���������
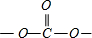 �У�Cԭ�ӳ�2��C-O���������ڦҼ���1��C=O˫����˫�����������㣬������Cԭ�ӵ��ӻ������Ϊ3����ȡsp2�ӻ���Ϊƽ���������Σ�����Ϊ120�㣬��O-C-O�ļ���ԼΪ 120�㣬
�У�Cԭ�ӳ�2��C-O���������ڦҼ���1��C=O˫����˫�����������㣬������Cԭ�ӵ��ӻ������Ϊ3����ȡsp2�ӻ���Ϊƽ���������Σ�����Ϊ120�㣬��O-C-O�ļ���ԼΪ 120�㣬�ʴ�Ϊ��MgO�����ӵĵ�ɶࡢ���Ӱ뾶��С�������ܴ�sp2��sp3��120�㣻
��4��F��Mg��K�γ���������ṹ�Ļ����������Fռ������������ģ�������Fԭ����ĿΪ12��
| 1 |
| 4 |
| 1 |
| 8 |
��5������Ԫ��K���ε���ɫ��ӦΪ��ɫ����������ζ����Է�����ɫ��Ӧ����ԭ����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�������
�ʴ�Ϊ���ϣ�����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�������
���������⿼��Ԫ���ƶϡ���������Ų����ɡ����ṹ�����ʡ���������ȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���Ĺؼ���Ҫ�������ԭ�������Ĺ�ϵ������ڱ��Ľṹ�����ж�Ԫ�أ�

��ϰ��ϵ�д�
�����Ŀ
