��Ŀ����
��Na2SO3����������ҵSO2β����Ⱦ������һ������SO2β����Ⱦ�Ƚ���ӱ�ķ�������������ǣ�
�ٽ�����SO2����Ĺ���β��ͨ�뵽Na2SO3��Һ�У���������β���е�SO2���壻
�ڽ�������SO2�������Һ���ȣ�����ʹ���е�SO2�����ݳ�����ЩSO2�������������ȡH2SO4��
(1)�÷�����Na2SO3��ѭ��ʹ�ã�������ɫ��ѧ�Ļ���ԭ�������������Ϣд���١��������Ļ�ѧ����ʽ��
��_______________________________��
��_______________________________��
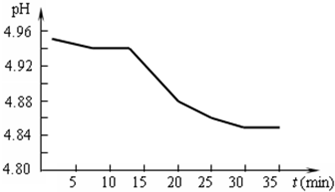
(2)����10 m3��0.1 mol/L��Na2SO3��Һ��ÿ�����ձ�״���µĺ���SO2�����β��89.6 m3���ﵽ����״̬�������T��������β����SO2�������������Ƕ��٣�
�ٽ�����SO2����Ĺ���β��ͨ�뵽Na2SO3��Һ�У���������β���е�SO2���壻
�ڽ�������SO2�������Һ���ȣ�����ʹ���е�SO2�����ݳ�����ЩSO2�������������ȡH2SO4��
(1)�÷�����Na2SO3��ѭ��ʹ�ã�������ɫ��ѧ�Ļ���ԭ�������������Ϣд���١��������Ļ�ѧ����ʽ��
��_______________________________��
��_______________________________��
(2)����10 m3��0.1 mol/L��Na2SO3��Һ��ÿ�����ձ�״���µĺ���SO2�����β��89.6 m3���ﵽ����״̬�������T��������β����SO2�������������Ƕ��٣�
(1) ��Na2SO3 +SO2+H2O = 2NaHSO3
��
(2)25%
��

(2)25%

��ϰ��ϵ�д�
�����Ŀ