��Ŀ����
����Ŀ��ij������Ʒ�к��������Ȼ������ʣ�������ͼ��ʾװ�����ⶨ�ô�����Ʒ�Ĵ��ȡ�ʵ�鲽�����£�
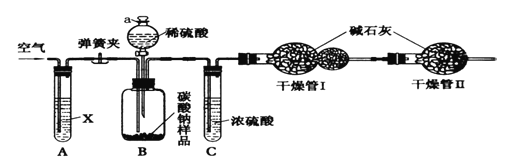
����ͼ��������װ�ò���������ԣ���ȷ����ʢ�м�ʯ�ң������������ƺ���ʯ�ҵĻ����ĸ����������������Ϊm1������ȷ����������Ʒ����������Ϊn����������ƿB�ڣ�����Һ©��a����������������ϡ���ᣬ�����ٲ�������Ϊֹ�������Թ�A����������������ӣ�Ȼ����������������������Ϊm2�����Իش�
��1��ʵ�������������Ҫ�����ĵؽ��У���������_____________���������������̫�죬�ᵼ�²ⶨ���________����ƫ��ƫС�䣩��
��2�����������Ŀ����______________��װ��A�е��Լ�XӦѡ��________________����������__________________��
��3��װ��C��������___________________�����������������__________________��
��4��װ��A��B֮��ĵ��ɼ��ڵ�_________�����ǰ������ڵ�_________�����������ǰ����н���
��5�����ݴ�ʵ�飬д�����㴿����Ʒ���ȵĹ�ʽ��___________________��
���𰸡���1��ʹ��Ӧ������CO2���ʯ�ҳ�ַ�Ӧ������ȫ���գ�ƫС��
��2��ʹ���ƿ�в�����CO2ȫ���ų���NaOH��Һ����ȥ�����к��е�CO2��
��3����ȥ�����е�ˮ��������ֹ�����е�CO2��ˮ������������I��
��4����������5��![]() ��100%
��100%
��������
�����������1����������ϡ���ᣬʹ��Ӧ���е���ȫ���������������ʹ�����Ķ�����̼ȫ�������գ������ʹһ���ֶ�����̼���������վͱ��ų������¶�����̼������ƫС��ʹ������ƫС�����Ա����Ϊ��ʹ��Ӧ������CO2���ʯ�ҳ�ַ�Ӧ������ȫ���գ�ƫС��
��2���������������ѹ����ʹ�����Ķ�����̼ȫ�����ų���Ҫ�ⶨ������̼�����������ų������ж�����̼�����ɵĶ�����̼��������Ӱ�죬��ȥ������̼ʹ�õ�������������Һ�����Ա����Ϊ��ʹ���ƿ�в�����CO2ȫ���ų���NaOH��Һ����ȥ�����к��е�CO2��
��3��C��ʢ�е���Ũ���ᣬ������ˮ�ԣ��ܽ�ˮ���������ų�ˮ�ֶ����ɶ�����̼������Ӱ�죬��������ڸ����I֮������ֹ�����еĶ�����̼��ˮ��������I�����Ա����Ϊ����ȥˮ��������ֹ�����е�CO2��ˮ������������I��
��4��A��B֮��ĵ��ɼ��ڹ������֮ǰ��رգ����������ʱ��Ҫ�����Ա����Ϊ����������
��5�������������ݣ����ɶ�����̼������Ϊ��m2-m1������̼���Ƶ�����Ϊx������
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
X m2-m1 106/x=44/( m2-m1)
���x=![]() ���Դ�����Ʒ����Ϊ��
���Դ�����Ʒ����Ϊ��![]() ��100%
��100%

 �����ܿ����ϵ�д�
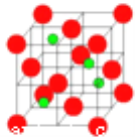
�����ܿ����ϵ�д�����Ŀ���±�������X��Y��Z��W���ֶ���������Ԫ�صIJ�����Ϣ��������Щ��Ϣ�ж�����˵������ȷ����(����)
Ԫ�� | X | Y | Z | W |
ԭ�Ӱ뾶(nm) | 0.102 | 0.16 | 0.074 | 0.071 |
������ۻ������ | ��6 | ��2 | ��2 | ��1 |
A. HW�����ȶ�����ǿ���⻯�� B. Z���������Ϊ��6
C. ԭ������X��Y��Z��W D. ������X�봿����Z��Ӧ����XZ3