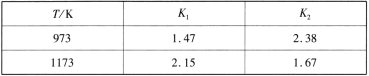
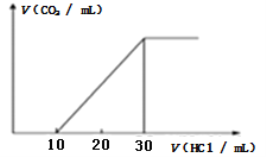
��Ŀ����
����Ŀ��ͨ��ѪҺ�еĸ����ӵļ���ܹ������ж϶��ּ�����ij�о�С��Ϊ�ⶨѪҺ��Ʒ��Ca2���ĺ���(100mLѪҺ�к�Ca2��������)��ʵ�鲽�����£�
��ȷ��ȡ5.00mLѪҺ��Ʒ�����������Ƴ�50.00mL��Һ��
��ȷ��ȡ��Һ10.00mL���������(NH4)2C2O4��Һ��ʹCa2����ȫת����CaC2O4������
�۹��˲�ϴ������CaC2O4�������ù���ϡ�����ܽ⣬����H2C2O4��CaSO4ϡ��Һ��
�ܼ���12.00mL0.0010mol��L��1��KMnO4��Һ��ʹH2C2O4��ȫ�����������ӷ���ʽΪ��2MnO4-��5H2C2O4��6H��=10CO2����2Mn2����8H2O��
����0.0020mol��L-1(NH4)2Fe(SO4)2��Һ�ζ�������KMnO4��Һ������(NH4)2Fe(SO4)2��Һ20.00mL��
��1����֪������CaC2O4��Ksp��2.0��10��9����ʹ�������c(Ca2��)��1.0��10��5mol��L��1��Ӧ������Һ��c(C2O42��)��_____mol��L-1��
��2�����������Mn2�����ɣ�������Ӧ�����ӷ���ʽΪ_____��
��3��������ݵζ�����ʹ��ǰδ�ñ�(NH4)2Fe(SO4)2��Һϴ�ӣ����ѪҺ��Ca2+�ĺ�����____(�ƫ�ߡ�����ƫ�͡����䡱)��
��4������Ѫ����Ca2���ĺ���_____(д���������)��
���𰸡�2.0��10-4 MnO4-��5Fe2����8H��=5Fe3����Mn2����4H2O ƫ�� 0.04g
��������
(1)�����ܶȻ�����Ksp��c(C2O42)c(Ca2��)�����㣻
(2)(NH4)2Fe(SO4)2��Һ�е�Fe2�����н�ǿ�Ļ�ԭ�ԣ��ܱ����Ը��������Һ����ΪFe3����
(3)���ݵζ�ԭ���ͷ���ʽ�жϣ�
(4)���ݹ�ϵʽ���㡣
(1)��Ksp��c(C2O42)c(Ca2��)��֪����ʹc(Ca2��)��1.0��105molL1��Ӧ������Һ��c(C2O42)��![]() ��
��![]() mol/L��2.0��104 mol/L���ʴ�Ϊ��2.0��104��
mol/L��2.0��104 mol/L���ʴ�Ϊ��2.0��104��
(2)(NH4)2Fe(SO4)2��Һ�е�Fe2�����н�ǿ�Ļ�ԭ�ԣ��ܱ����Ը��������Һ����ΪFe3�������ӷ���ʽΪ��MnO4-��5Fe2����8H��=5Fe3����Mn2����4H2O���ʴ�Ϊ��MnO4-��5Fe2����8H��=5Fe3����Mn2����4H2O��
(3)������ݵζ�����ʹ��ǰδ�ñ�(NH4)2Fe(SO4)2��Һϴ�ӣ�����ζ��ܱ�������ˮ��ע��ı�(NH4)2Fe(SO4)2��Һ�ᱻϡ�ͣ����ĸ���ı�(NH4)2Fe(SO4)2��Һ�����ʣ��KMnO4��Һƫ�࣬KMnO4��Һ����H2C2O4ƫ�٣����Բ��ѪҺ��Ca2���ĺ�����ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
(4)KMnO4�������ʵ���Ϊ��0.0010 molL1��12��103L��1.2��105 mol��(NH4)2Fe(SO4)2��Һ�ζ����ĵĹ�����KMnO4�����ʵ���Ϊ��0.0020 molL1��20.00��103L��![]() ��8.0��106 mol����H2C2O4��Ӧ��KMnO4����Ϊ��1.2��105 mol8.0��106 mol��4.0��106 mol��n(H2C2O4)��4.0��106 mol��
��8.0��106 mol����H2C2O4��Ӧ��KMnO4����Ϊ��1.2��105 mol8.0��106 mol��4.0��106 mol��n(H2C2O4)��4.0��106 mol��![]() ��1.0��105 mol��n(CaC2O4)��1.0��105 mol������100 mLѪ����Ca2���ĺ���Ϊ��1.0��105 mol��40 gmol1��
��1.0��105 mol��n(CaC2O4)��1.0��105 mol������100 mLѪ����Ca2���ĺ���Ϊ��1.0��105 mol��40 gmol1��![]() ��
��![]() ��0.04g��
��0.04g��
��100mlѪ����Ca2���ĺ���Ϊ0.04g��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�