��Ŀ����
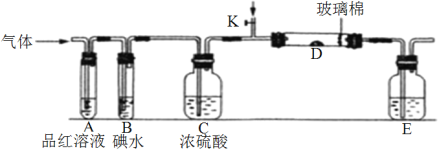
����Ŀ����Ԫ���γɵĻ���������ʮ�ַḻ����������¹�ҵ�������ԭ��ʾ��ͼ�ش���������ص����⣺

��1�������й�NH3��˵���У�����ȷ����__________(˫ѡ)��
A.��ҵ�ϳ�NH3��Ҫ�ڸ��¸�ѹ�½��� B. NH3����������̼狀����صȻ���
C. NH3����Ũ�������ˮ�Ȼ��Ƹ��� D. NH3�����ֽ⣬�������䰵������
��2��NH3����ˮ����״���£��ó���NH3����ƿ����Ȫʵ�飬ˮ����������ƿ�����γ���Һ�����ʵ���Ũ��Ϊ________________mol.L-1��
��3������������¯����������Ӧ�Ļ�ѧ����ʽΪ_____________________________________________��
��4������������ β�����к�NO��NO2�ȵ��������β���ų���Ϊ�������ǶԻ������ƻ����ã�ͨ�����������ַ���������
�ٴ�����Һ���շ���������Һ��NO2�ķ�Ӧԭ��Ϊ��Na2CO3+2NO2=NaNO3+________+CO2 (����д��ɻ�ѧ����ʽ)��
�ڰ�ת��������֪7mol��ǡ���ܽ���NO��NO2��6mol�Ļ��������ȫת��ΪN2,����������NO��NO2�����ʵ���֮��Ϊ__________��
���𰸡�CD1/22.44NH3+5O2![]() 4NO+6H2ONaNO21:3
4NO+6H2ONaNO21:3
��������
(1)A. �ϳɰ����ڸ��¸�ѹ�����½��У�����ȷ��B.����������ȡ̼狀����أ�����ȷ��C.�����ܱ�Ũ�������գ��ʴ���D.���������ֽ⣬�ʴ���ѡCD�� (2)���谱�������Ϊ1L�����������ʵ���Ϊ1/22.4mol��������������ˮ���������õ�����Һ�����Ϊ1L������Һ�����ʵ���Ũ��Ϊ��1/22.4��/1=1/22.4mol/L�� (3)�����̷�����������������Ӧ����һ����̼��ˮ������ʽΪ��4NH3+5O2![]() 4NO+6H2O��(4)ͨ�������غ��������ȱ��һ����ԭ�Ӻ�һ����ԭ�Ӻ�������ԭ�ӣ����Բ���ΪNaNO2�� (5)����һ�����������ʵ���Ϊxmol���������������ʵ���Ϊymol������ת�Ƶ�������ȷ�����2x+4y=7��3 x+y=6����x=1.5 y=4.5����һ�������Ͷ������������ʵ�����Ϊ1.5:4.5=1:3��
4NO+6H2O��(4)ͨ�������غ��������ȱ��һ����ԭ�Ӻ�һ����ԭ�Ӻ�������ԭ�ӣ����Բ���ΪNaNO2�� (5)����һ�����������ʵ���Ϊxmol���������������ʵ���Ϊymol������ת�Ƶ�������ȷ�����2x+4y=7��3 x+y=6����x=1.5 y=4.5����һ�������Ͷ������������ʵ�����Ϊ1.5:4.5=1:3��

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�����Ŀ��ijУ��ȤС���SO2������Cu(OH)2����Һ�ķ�Ӧ����̽����ʵ�����£�
װ�� | ��� | �Թ��е�ҩƷ | ���� |
����ͨ��
| ʵ��� | 1.5 mL1 mol/L CuSO4��Һ��3.5 mL 1mol/L NaOH��Һ��� | ��ʼʱ��ש��ɫ�������֣�һ��ʱ���ש��ɫ������ʧ�����ã��Թܵײ��������Ϻ�ɫ���壬��Һ����ɫ |
ʵ��� | 1.5 mL1 mol/L CuCl2��Һ��3.5 mL 1 mol/L NaOH��Һ��� | ��ʼʱ�л�ɫ�������֣�һ��ʱ���ɫ������ʧ�����ã����ɴ�����ɫ��������Һ����ɫ |
��1����ȡ����Cu(OH)2����Һ�����ӷ���ʽΪ__________________________________��
��2����ͬѧ������ʵ��II�ķ����Ʊ�����Cu(OH)2����Һ�����ˣ�������ˮϴ�Ӹɾ�����ϴ�����Cu(OH)2�м���5 mL����ˮ���ٳ���ͨ��SO2���壬������ʵ��I��ͬ���˲�ʵ��֤����__________________������Cu(OH)2ϴ�Ӹɾ��ķ�����____________________________________________��
��3��ͬѧ�Ƕ�ɫ�����ijɷּ�������̽���������������£�CuClΪ��ɫ���壬������ˮ��������Ũ���ᡣ���백ˮ��Ӧ����Cu(NH3)2+���ڿ����л������������ɺ�����ɫCu(NH3)42+��Һ��
�ټ�ͬѧ��ϴ�ӵõ��İ�ɫ�����м��백ˮ���õ���ɫ��Һ���˹����з�Ӧ�����ӷ���ʽΪ��CuCl+2NH3��H2O
=Cu(NH3)2++Cl-+2H2O��____________________________��
����ͬѧ����һ�ַ���֤���˸ð�ɫ����ΪCuCl���ο�����ʵ�鷽����д����

��д�±��ո�
�Լ�1 | _________________ | �Լ�2 | ����ˮ |
����1 | _________________ | ����2 | _________________ |
��д��ʵ��II����Cu(OH)2���ɰ�ɫ���������ӷ���ʽ��_________________��
��4����ͬѧͨ��ʵ��֤����ʵ����й۲쵽��ש��ɫ������Cu2O����ɺ�����ʵ�鷽����ȡ����Cu2O�������Թ��У�_________________________________����˵��ש��ɫ������Cu2O��