��Ŀ����
ij�����廯����A������ֻ��һ��ȡ����,�����ķ�Ӧ��ͼ��ʾ,B��F��G�ķ���ʽ�ֱ�ΪC2H4O2��H2C2O4��C6H12O,H��ʹ��ˮ��ɫ��
(1)A�Ľṹ��ʽ ,H�ļ���ʽ ��
(2)B�еĹ���������Ϊ ,�ݵķ�Ӧ����Ϊ ��
(3)C��һ���������γɸ߷��ӻ�����Ļ�ѧ����ʽ ��
(4)����G��F��Ӧ�Ļ�ѧ����ʽ ��
(5)G��ͬ���칹�����ܷ���������Ӧ�Ĺ��� ��,���к˴Ź���������������������л���ṹ��ʽΪ ��
(1)

(2)�Ȼ� ��ȥ��Ӧ
(3)nHOCH2COOH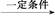
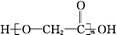 +(n-1)H2O
+(n-1)H2O
(4)2 +HOOCCOOH
+HOOCCOOH
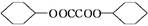 +2H2O
+2H2O
(5)8 (CH3)3CCH2CHO
����

ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ����ʣ�ʵ��װ������ͼ��ʾ��
���Ⱦ۱�ϩ�����ϵõ��IJ������±���
| ���� | ���� | ���� | ��ϩ | ��ϩ | �� | �ױ� | ̼ |
| ��������/% | 12 | 24 | 12 | 16 | 20 | 10 | 6 |
(1)�Թ�A�в������ж�����;��������ת���Ϳ���ȡ�߾������Ȳ��
A�����
 ����Ȳ
����Ȳд����Ӧ�ڢ۵Ļ�ѧ����ʽ��__________________________��
(2)B���ռ��õ�����������ʹ����KMnO4��Һ��ɫ�����ʵ�һ�ȴ�����________�֡�
(3)��ƿC�й۲쵽��������______________������ˮ������գ�ʣ�����徭�����ƽ����Է�������Ϊ________��
(4)д��C���ݳ��������ڹ�ҵ�ϵ�������;__________��____________��
ij�������Ա�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�����ҩ����������

�ش��������⣺
��1�����������ӣ�����˵����ȷ����________��
| A��1 mol�����������Ժ�2 mol NaOH��Ӧ |
| B��������������Ӧ |
| C���ɷ���ˮ�ⷴӦ |
| D�������巢��ȡ����Ӧ |
��3��д��B��C�Ļ�ѧ����ʽ__________________________________________��
��4��д��������F�Ľṹ��ʽ__________��
��5��д��ͬʱ��������������F��ͬ���칹��Ľṹ��ʽ________________________________________________________��д��3�֣���
�����������ұ����������ֲ�ͬ��ѧ��������ԭ�ӡ�
���ܷ���������Ӧ��
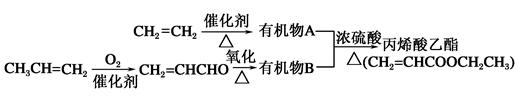
��6���Ա�����ϩΪԭ�Ͽɺϳɾ۱���ϩ������ƺϳ�·�ߣ����Լ����ܼ���ѡ��
ע���ϳ�·�ߵ���д��ʽ��������ʾ������ͼ��
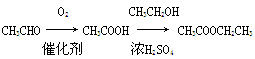



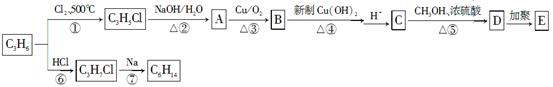
 ��ͨ����ϩ���Ƶ�E���乤ҵ�ϳ�·����ͼ��ʾ��
��ͨ����ϩ���Ƶ�E���乤ҵ�ϳ�·����ͼ��ʾ��
 2CH2=CHCN��6H2O
2CH2=CHCN��6H2O

 ��C10H8�������������ܾ��еĻ�ѧ������________��ѡ���ţ���
��C10H8�������������ܾ��еĻ�ѧ������________��ѡ���ţ���
