题目内容
(18分)铵盐在工农业生产中有着重要的用途,请根据要求完成下列各题。
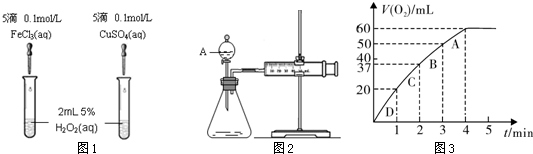
Ⅰ.某化学兴趣小组欲从下列装置中选取必要的装置制取(NH4)2SO4溶液。
(1)仪器连接的顺序(用接口序号字母表示)是:a
(2)试从电离平衡角度分析该实验装置A中能产生氨气的原因:
。
(3)将装置C中两种液体分离开的操作名称是 。
(4)(NH4)2SO4“低毒,有刺激性,有吸湿性、吸湿后固结成块”。储存应注意 。
Ⅱ.为提高氯化铵的经济价值,我国化学家设计了利用氢氧化镁热分解氯化铵制氨气并得到碱式氯化镁[Mg(OH)Cl]的工艺。某同学根据该原理设计的实验装置如图: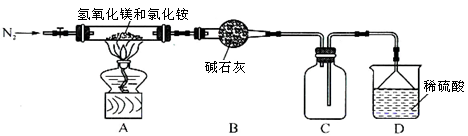
请回答下列问题:
(1)装置A中发生反应生成碱式氯化镁的化学方程式为 。
(2)反应过程中持续通入N2的作用有两点:一是使反应产生的氨气完全导出并被稀硫酸充分吸收,
二是 。
(3)由MgCl2溶液蒸发得到MgCl2·6H2O晶体,蒸发的目的是_________。
a.得到热饱和溶液 b.析出晶体
(4)镁是一种用途很广的金属材料,目前世界上60%的镁从海水中提取。
①若要验证所得无水MgCl2中不含NaCl,最简单的操作方法是:
。
②由MgCl2·6H2O制备无水MgCl2的操作在 氛围中进行,若在空气中加热,则会生成Mg(OH)Cl。
(18分)
Ⅰ(1)d e f (2分)
(2)氢氧化钠电离出的OH-增大了氨水中OH-浓度,促使氨水电离平衡NH3+H2O  NH4++OH—左移,有利氨气逸出。(2分)
NH4++OH—左移,有利氨气逸出。(2分)
(3) 分液(2分)。
(4)密封、阴凉、通风(2分)
Ⅱ(1)Mg(OH)2+NH4Cl MgOHCl+NH3↑+H2O(2分)
MgOHCl+NH3↑+H2O(2分)
(2)防止装置C中的AlCl3溶液倒吸入装置B(2分)
(3)a(2分)
(4)①用铂丝蘸取少量固体,置于酒精灯火焰上灼烧,若无黄色火焰产生,则证明所得无水氯化镁晶体中不含氯化钠。(2分)(答焰色反应也给分)
②HCl(气流)(2分)
解析试题分析:Ⅰ(1)制取的氨气与硫酸反应生成硫酸铵,剩余的氨气用水吸收,故连接顺序为a→d→e→f。
(2)氨水中存在电离平衡: NH3+H2O  NH4++OH—,与NaOH混合后,NaOH电离出OH-,促使氨水电离平衡向左移动,有利氨气逸出。
NH4++OH—,与NaOH混合后,NaOH电离出OH-,促使氨水电离平衡向左移动,有利氨气逸出。
(3)分离互不相溶的液体的方法是:分液。
(4)因为(NH4)2SO4“低毒,有刺激性,有吸湿性、吸湿后固结成块”,应防止水分进入,所以储存应注意密封、阴凉、通风。
Ⅱ(1)根据题目所给信息,反应物为Mg(OH)2和NH4Cl,生成物为MgOHCl、NH3和H2O,所以化学方程式为:Mg(OH)2+NH4Cl MgOHCl+NH3↑+H2O。
MgOHCl+NH3↑+H2O。
(2)防止装置C中的AlCl3溶液倒吸入装置B
(3)由MgCl2溶液得到MgCl2·6H2O晶体的方法为蒸发浓缩、降温结晶,所以蒸发的目的是:得到热饱和溶液,故a项正确。
(4)①Na元素用焰色反应进行鉴别,所以操作方法是:用铂丝蘸取少量固体,置于酒精灯火焰上灼烧,若无黄色火焰产生,则证明所得无水氯化镁晶体中不含氯化钠。
②因为MgCl2为强酸弱碱盐,Mg2+发生水解反应,在HCl(气流)中可以抑制MgCl2的水解,所以由MgCl2·6H2O制备无水MgCl2的操作在HCl氛围中进行。
考点:本题考查实验基本操作、实验方案的分析、物质的检验、平衡移动、盐类的水解。

某酸性工业废水中含有K2Cr2O7。光照下,草酸(H2C2O4)能将其中的Cr2O72—转化为Cr3+。某课题组研究发现,少量铁明矾[Al2Fe(SO4)4·24H2O]即可对该反应起催化作用。为进一步研究有关因素对该反应速率的影响,探究如下:
(1)在25 ℃下,控制光照强度、废水样品初始浓度和催化剂用量相同,调节不同的初始pH和一定浓度草酸溶液用量,做对比实验,完成以下实验设计表(表中不要留空格)。
| 实验编号 | 初始pH | 废水样品体积/mL | 草酸溶液体积/mL | 蒸馏水体积/mL |
| ① | 4 | 60 | 10 | 30 |
| ② | 5 | 60 | 10 | 30 |
| ③ | 5 | 60 | | |
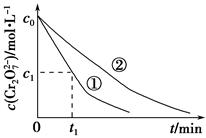
测得实验①和②溶液中的Cr2O72—浓度随时间变化关系如图所示。
(2)上述反应后草酸被氧化为________(填化学式)。
(3)实验①和②的结果表明________;实验①中0~t1时间段反应速率v(Cr3+)=________mol·L-1·min-1(用代数式表示)。
(4)该课题组对铁明矾[Al2Fe(SO4)4·24H2O]中起催化作用的成分提出如下假设,请你完成假设二和假设三:
假设一:Fe2+起催化作用:
假设二:________;
假设三:________;
……
(5)请你设计实验验证上述假设一,完成下表中内容。
(除了上述实验提供的试剂外,可供选择的药品有K2SO4、FeSO4、K2SO4·Al2(SO4)3·24H2O、Al2(SO4)3等。溶液中Cr2O72—的浓度可用仪器测定)
| 实验方案(不要求写具体操作过程) | 预期实验结果和结论 |
某探究小组用测量HNO3与大理石反应过程中质量减小的方法,研究影响反应速率的因素。限选试剂:1.00 mol·L-1 HNO3、2.00 mol·L-1 HNO3,细颗粒大理石、粗颗粒大理石,35 ℃水浴。
(1)他们能完成哪些因素对速率影响的探究?
____________________________________________________________。
(2)请根据能进行的探究内容,填写以下实验设计表,完成探究实验:
(3)整个实验中应控制的不变量是硝酸溶液体积和 。
(4)该实验小组用如图实验装置进行实验。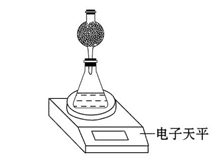
①除电子天平、干燥管、锥形瓶、药匙、胶塞等仪器外,必需的实验仪器还有 。
②干燥管中应放置的试剂是 。
| A.碱石灰 | B.无水CaCl2 |
| C.P2O5固体 | D.浓硫酸 |
甲酸甲酯水解反应方程式为:HCOOCH3 + H2O  HCOOH + CH3OH – Q(Q>0)
HCOOH + CH3OH – Q(Q>0)
某小组通过实验研究该反应(反应过程中体积变化忽略不计)。反应体系中各组分的起始量如下表。甲酸甲酯转化率在温度T1下随反应时间(t)的变化如下图:
| 组分 | 物质的量/mol |
| HCOOCH3 | 1.00 |
| H2O | 1.99 |
| HCOOH | 0.01 |
| CH3OH | 0.52 |

(1)上述反应的平衡常数表达式为K=_______________________。
(2)计算15~20min范围内:甲酸甲酯的减少量为 mol,甲酸甲酯的平均反应速率为 mol/min;80~90min范围内甲酸甲酯的平均反应速率为___________ mol/min。
(3)依据以上数据,推断该反应在10min后反应速率迅速加快的原因: 。
(4)其他条件不变,提高温度为T2,在答题卡框图中画出温度T2下甲酸甲酯转化率随反应时间变化的预期结果示意图。
0.02mol·L—1的HCN溶液与0.02mol·L—1的NaCN溶液等体积混合,已知混合溶液中C(CN—)<C(Na+),则下列关系中,正确的是( )
| A.C(Na+)>C(CN—)>C( H+)>C(OH―) | B.C(HCN)+C (CN—)=0.04mol·L—1 |
| C.C(Na+)+C(H+)= C(CN—)+C(OH―) | D.C(CN—)>C(HCN) |
 SO2(g)+Cl2(g) △H=+97.3 kJ·mol-1。某温度时向体积为1 L的恒容密闭容器中充入0. 20mol SO2Cl2,达到平衡时,容器中含0.18mol SO2,则此过程反应吸收的热量为_____ kJ,该温度时反应的平衡常数为_____。将上述所得混合气体溶于足量BaCl2溶液中,最终生成沉淀的质量为_______。
SO2(g)+Cl2(g) △H=+97.3 kJ·mol-1。某温度时向体积为1 L的恒容密闭容器中充入0. 20mol SO2Cl2,达到平衡时,容器中含0.18mol SO2,则此过程反应吸收的热量为_____ kJ,该温度时反应的平衡常数为_____。将上述所得混合气体溶于足量BaCl2溶液中,最终生成沉淀的质量为_______。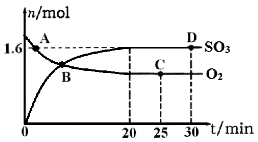
 CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)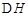 =-49.0kJ·mol
=-49.0kJ·mol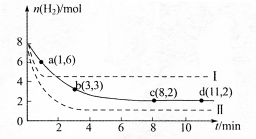
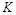 ,曲线Ⅰ对应条件下平衡常数为
,曲线Ⅰ对应条件下平衡常数为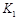 ,曲线Ⅱ对应条件下平衡常数为
,曲线Ⅱ对应条件下平衡常数为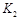 ,则
,则