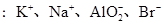��Ŀ����
�������������������γ��������Ҫ���ʡ�ij�������п��ܺ����������ӣ�Na����Ba2����NH4+��Al3����Cl����SO32-��SO42-��NO3-�ȡ�ij�о�С��ȡ�õ�һ���������꣬Ũ�������ó�����Һ�ֳ����ݣ���������ʵ�飺
��ش��������⣺
(1)����ʵ�����жϸ������п϶������ڵ�������______________������ȷ����������________________��
(2)д����һ����Һ�μӵ���KI��Һʱ������Ӧ�����ӷ���ʽ��__________________��
(3)��������Һ�μ�NaOH��Һ�����ȣ����������з����˶����Ӧ��д������������Ӧ�����ӷ���ʽ��__________________________________��__________________________��
(4)���ʵ�鷽����������������Ƿ����Cl����___________________________________
______________________________��
(5)��С��Ϊ��̽��NO����������������γɹ��̣�����ƿ�г��뺬������NO��SO2���壬������ͨ��O2��������ѧ��Ӧ����������������ˮ�������������꣬��NO��������Ӧ�е�������________________________________________________________��
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ��Һ����ɫ |
| �ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ�������� |
| ��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ���(V)�����ɵij�������������������ʵ���(n)�Ĺ�ϵ����ͼ | 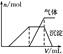 |
��ش��������⣺
(1)����ʵ�����жϸ������п϶������ڵ�������______________������ȷ����������________________��
(2)д����һ����Һ�μӵ���KI��Һʱ������Ӧ�����ӷ���ʽ��__________________��
(3)��������Һ�μ�NaOH��Һ�����ȣ����������з����˶����Ӧ��д������������Ӧ�����ӷ���ʽ��__________________________________��__________________________��
(4)���ʵ�鷽����������������Ƿ����Cl����___________________________________
______________________________��
(5)��С��Ϊ��̽��NO����������������γɹ��̣�����ƿ�г��뺬������NO��SO2���壬������ͨ��O2��������ѧ��Ӧ����������������ˮ�������������꣬��NO��������Ӧ�е�������________________________________________________________��
(1)SO32-��Ba2����Na����Cl��
(2)6I����2NO3-��8H��=2NO����3I2��4H2O
(3)H����OH��=H2O��NH4+��OH�� NH3����H2O��Al3����3OH��=Al(OH)3����Al(OH)3��OH��=AlO2-��2H2O(�����)
NH3����H2O��Al3����3OH��=Al(OH)3����Al(OH)3��OH��=AlO2-��2H2O(�����)
(4)ȡ������Һ�μ�������Ba(NO3)2��Һ������ȡ�ϲ����Һ����HNO3�ữ��AgNO3��Һ�����а�ɫ�������ɣ���֤������Cl��
(5)����
(2)6I����2NO3-��8H��=2NO����3I2��4H2O
(3)H����OH��=H2O��NH4+��OH��
 NH3����H2O��Al3����3OH��=Al(OH)3����Al(OH)3��OH��=AlO2-��2H2O(�����)
NH3����H2O��Al3����3OH��=Al(OH)3����Al(OH)3��OH��=AlO2-��2H2O(��д����)(4)ȡ������Һ�μ�������Ba(NO3)2��Һ������ȡ�ϲ����Һ����HNO3�ữ��AgNO3��Һ�����а�ɫ�������ɣ���֤������Cl��
(5)����
(1)�ɵ�һ����Һ����������֪�������к���NO3-��������������SO32-������NO3-���棬����Һ��û��SO32-���ɵڶ�����Һ����������֪�������к���SO42-��û��Ba2�����ɵ�������Һ����������֪�������к���Al3����NH4+���ʸ������п϶������ڵ�������SO32-��Ba2��������ȷ����������Na����Cl����(2)�μӵ���KI��Һʱ��I��������ΪI2��NO3-����ԭΪNO��(3)��������Һ��OH���ֱ���H����Al3����NH4+��Al(OH)3������Ӧ��(4)����Cl��ʱ����Ҫ����������Ba(NO3)2��Һ��SO42-��ȥ���ų����ţ�Ȼ����HNO3�ữ��AgNO3��Һ���м��顣(5)NO�ܱ�������NO2��NO2��ˮ��ת��ΪHNO3��ϡHNO3����SO32-���յõ��������������꣬���NO�������γɹ�������������

��ϰ��ϵ�д�
�����Ŀ

 ��Ba2+��OH-��I-
��Ba2+��OH-��I-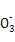
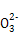 ��Cl-
��Cl-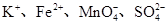
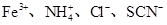
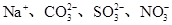
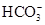 ����Һ��
����Һ��