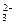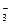��Ŀ����
��6�֣�����������Ԫ��A��B��C��D��ԭ������������������A��Cͬ���壬B��C��Dͬ���ڣ�Aԭ�ӵ������������Ǵ�����������3����B�Ƕ�����Ԫ����ԭ�Ӱ뾶��������Ԫ�ء��Իش��������⣺
(1)A��Ԫ�ط���______________��D��ԭ�ӽṹʾ��ͼ______________��
(2)A��B��C����Ԫ���γɵļ����ӵİ뾶�ɴ�С��˳����_______________________��
(3)A��B��C��D�γɵĻ�����B2A2��CD2��D2A��DA2�и�ԭ�Ӷ����������8���ӽṹ����______________(��д����Ļ�ѧʽ )��
(4)CA2��DԪ�صĵ�����ˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ��______________________________��
(1)A��Ԫ�ط���______________��D��ԭ�ӽṹʾ��ͼ______________��
(2)A��B��C����Ԫ���γɵļ����ӵİ뾶�ɴ�С��˳����_______________________��
(3)A��B��C��D�γɵĻ�����B2A2��CD2��D2A��DA2�и�ԭ�Ӷ����������8���ӽṹ����______________(��д����Ļ�ѧʽ )��
(4)CA2��DԪ�صĵ�����ˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ��______________________________��
��1��O 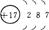
��2��S2-> O2-> Na+
��3��Na2O2,SCl2,Cl2O
��4��SO2+Cl2+2H2O====2HCl+H2SO4
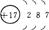
��2��S2-> O2-> Na+
��3��Na2O2,SCl2,Cl2O
��4��SO2+Cl2+2H2O====2HCl+H2SO4
��1��������֪AΪ��Ԫ��,BΪ��Ԫ�أ�C��Aͬ���壬��CΪ��Ԫ�أ���A��B��C��D��ԭ��������������Dֻ��Ϊ��Ԫ�ء���2��ͬ������ϵ��������Ӱ뾶����S2-> O2-�����Ӳ��Ų���ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС����O2-> Na+��
��3��������Ԫ���У�����H��He�� Li��Be��B�⣬�γɻ�����ʱ����㶼��8�����ȶ��ṹ��
��4��Cl2�������ԣ���SO2�л�ԭ�ԣ��ʶ��߷���������ԭ��Ӧ��
��3��������Ԫ���У�����H��He�� Li��Be��B�⣬�γɻ�����ʱ����㶼��8�����ȶ��ṹ��
��4��Cl2�������ԣ���SO2�л�ԭ�ԣ��ʶ��߷���������ԭ��Ӧ��

��ϰ��ϵ�д�
 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
�����Ŀ