��Ŀ����
����Ŀ������ֲ���纣���ͺ����к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() �D��
�D��![]()
��֪������������Ӧ�Ļ�ѧ����ʽΪCl2��2KI===2KCl��I2

ij��ѧ��ȤС�齫�������̢ڢ���Ƴ�����ͼ��ʾ������
�ش��������⣺
��1��д����ȡ���̢�������ʵ����������ƣ���_____________ ��_____________
��2��F���²�Һ�����ɫΪ_____ɫ���ϲ�Һ�������ʵ���Ҫ�ɷ�Ϊ_____�����ѧʽ��
��3�������չ����У�ʹ�õ���ʵ��������(����������)_______________(�����)��
���Թ� ������ ������ǯ �������� ���ƾ���
��4��Ϊ��ȡ����⣬����ȤС�������������װ��ͼ��
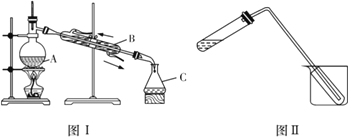
��ͼ��������A��C�����Ʒֱ���________��________����װ���еĴ���֮��Ϊ___________��
��ʵ��ʱ����A�г�����һ�������л���Һ�⣬�����������________����������_________ ��
��ͼ��װ�ÿ�������������ˮ����ȡ(���ȼ��̶�������)����ԭ����ͼ����ȫ��ͬ����װ����ʹ�õIJ������ܽϳ�����������_________���ձ��л�Ӧʢ�е�������_________________��
���𰸡� ���� ��ȡ �Ϻ� KCl �ڢۢ� ������ƿ ��ƿ ����ˮӦ���¿ڽ����Ͽڳ� ��ʯ ������ ����ˮ���� ��ˮ�����
����������������������ԴӺ�������ȡ�������Ϊ���壬������ˡ���ȡ��Һ������Ȼ���ʵ�������
��1�����̢��Ǵ�����Һ�л�ú�I-����Һ�����̢ٵIJ���Ϊ���ˡ����̢��Ǽ���CCl4����I2����Һ����ȡ��I2�����̢۵IJ���Ϊ��ȡ��
��2������CCl4���ܶȱ�ˮ���ܶȴ�F���²�Һ��ΪI2��CCl4��Һ����ɫΪ�Ϻ�ɫ���������е���Ҫ��ӦΪCl2+2KI=2KCl+I2����CCl4��ȡI2���ϲ�Һ�������ʵ���Ҫ�ɷ�ΪKCl��
��3�������չ����У�ʹ�õ���ʵ��������(����������)�ƾ��ơ�����������ǯ�����żܣ���ѡ�ڢۢ���
��4���ٸ��������Ľṹ�ص㣬ͼI������AΪ������ƿ������CΪ��ƿ��ͼIΪ����װ�ã���װ���еĴ���֮��Ϊ����ˮӦ���¿ڽ����Ͽڳ���
��ʵ��ʱ����A�г�����һ�������л���Һ�⣬�������������ʯ�������Ƭ�����������Ƿ�ֹҺ�����ʱ�ľ����������������С�
�۸�װ����ʹ�õIJ������ܽϳ���������������ˮ�������ձ��л�Ӧʢ�е������DZ�ˮ����Ŀ���dz�������������ˮ��

����Ŀ��ij�о���ѧϰС�����ʵ����30mLŨ������10mL��ˮ�Ҵ������Ʊ���ϩ���塢���ⶨ�Ҵ�ת������ϩ��ת���ʡ���֪���ɵ���ϩ�����к���SO2��CO2���Ҵ������ѵ����ʡ��й��������£�
�۵�/�� | �е�/�� | �ܽ��� | ��ɫ״̬ | �ܶ�g/cm3 | |
�Ҵ� | -114.1 | 78.3 | ��ˮ���л��ܼ����� | ��ɫҺ�� | 0.79 |
���� | -116.2 | 34.5 | ������ˮ���������л��ܼ� | ��ɫҺ�� | 0.7135 |
��1���Ʊ���ϩ
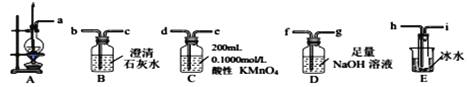
�� ��A��E��ѡ���Ҫ��װ�����ʵ�飬���������������ӵ�˳��Ϊ________���������ӿڵ���ĸ��ţ���
�� Dװ���з�����Ӧ�����ӷ���ʽ______ʵ�����D��Һ�к���CO32-��ʵ�鷽��Ϊ_____��
�� Eװ�õ���Ҫ������__________________��
��2���ⶨ��ϩ��Ӧ����������Һ����ȡC����Һ20mL����������Һ����仯������ƿ�У��ȼ���Լ2mLϡ�����ữ������0.1000mol/L��Na2C2O4��Һ�ζ�δ��Ӧ���KMnO4��
��֪��C2H4 ![]() CO2+H2O��C2O42-
CO2+H2O��C2O42- ![]() CO2+H2O��MnO4-��Mn2+
CO2+H2O��MnO4-��Mn2+
�� �������ʹ�òⶨ��ϩ����ƫ�ߵ���______
A����������ˮ��ϴ��ʽ�ζ��ܺ�ֱ��װNa2C2O4��Һ
B.��ƿ��ϴ�ɾ�����д���ˮ��
C.�ζ�ǰ���ζ����������ݣ��ζ���������
D������ʱ���ζ�ǰƽ�ӣ��ζ�����
�� �ζ��յ������Ϊ_______________��
�� ��֪��ȥNa2C2O4��Һ20.00mL�����Ҵ�ת������ϩ��ת����Ϊ_________����С�������2λ���֣�
