��Ŀ����
����Ŀ���л���A�Ľṹ��ʽΪ![]() ������ͨ�����仯ѧ��Ӧ�քe�Ƶ�B��C��D��E�������ʡ�
������ͨ�����仯ѧ��Ӧ�քe�Ƶ�B��C��D��E�������ʡ�

�ش��������⣺
(1)A�к��й����ŵ�������_____________�� A����B�ķ�Ӧ����Ϊ______________��
(2)��AE���������У���Ϊͬ���칹�����(����)_____________��
(3)����B�����ʣ�����˵����ȷ����________________(���ţ�
a.1 mol B������5mol H2�����ӳɷ�Ӧ
b.��������Cu(OH)2��Ӧ���ɺ�ɫ����
c.�ڴ��������£��ɱ������е���������
d.�÷��������е�ԭ����ͬһƽ����
(4) C���γɸ߾���ø߾���Ľṹ��ʽΪ_____________��
(5)д�����з�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ����)��
��A����C��_________________��
��D��NaOH��Һ���ȣ�__________________��
���𰸡� �ǻ����Ȼ� ������Ӧ C �� E bc 
![]()
��������(1) A�Ľṹ��ʽΪ![]() ��A�к��й�����Ϊ�ǻ����Ȼ���BΪ
��A�к��й�����Ϊ�ǻ����Ȼ���BΪ![]() ��������A�е��ǻ������õ����ʴ�Ϊ���ǻ����Ȼ���������Ӧ��
��������A�е��ǻ������õ����ʴ�Ϊ���ǻ����Ȼ���������Ӧ��
(2)�Ա������������ʣ�C��E�ķ���ʽ��ͬ����ΪC9H8O2�����ṹ��ͬ������ͬ���칹�壬�ʴ�Ϊ��C��E��
(3) BΪ![]() ��a.B�еı�����ȩ���ܹ��������ӳɣ�1 mol B������4mol H2�����ӳɷ�Ӧ������b.����ȩ������������Cu(OH)2��Ӧ���ɺ�ɫ��������ȷ��c.����ȩ�����ڴ��������£��ɱ������е�������������ȷ��d.�����Ǽ����÷��������е�ԭ�Ӳ�������ͬһƽ���ϣ�����ѡbc��
��a.B�еı�����ȩ���ܹ��������ӳɣ�1 mol B������4mol H2�����ӳɷ�Ӧ������b.����ȩ������������Cu(OH)2��Ӧ���ɺ�ɫ��������ȷ��c.����ȩ�����ڴ��������£��ɱ������е�������������ȷ��d.�����Ǽ����÷��������е�ԭ�Ӳ�������ͬһƽ���ϣ�����ѡbc��
(4)C�к���C=C���ɷ����Ӿ۷�Ӧ�����ɸ߾���Ϊ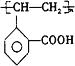 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
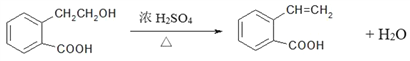
(5)��CΪ![]() ��������A�����ǻ�����ȥ��Ӧ���ɣ���Ӧ�Ļ�ѧ����ʽΪ
��������A�����ǻ�����ȥ��Ӧ���ɣ���Ӧ�Ļ�ѧ����ʽΪ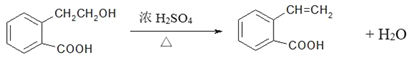 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��D�к���-COOH��-COO-��-COOH��NaOH�����кͷ�Ӧ��-COO-��NaOH��Һ��ˮ�⣬��Ӧ�ķ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
