��Ŀ����
�������Ʊ��治�����ױ��ʡ�
��1��ij����������Ʒ�Ѿ����ֱ��ʣ��������ʵ�飬����һ����Һ��֤�����������Ѿ����� ��
��2��������Ʒ�л��й������Ƶķ����� ��
��3��ij����С��Ϊ�˴��Բⶨ�������Ƶ��������������dz�ȡag��Ʒ������
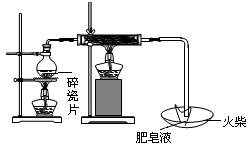
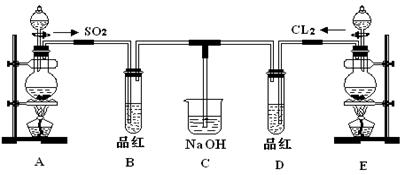
������ͼװ�����ⶨ�������Ƶ�����������
 ��#��#��#Դ��ͼ�е�E��F��������װ�ã������ⶨO2�������
��#��#��#Դ��ͼ�е�E��F��������װ�ã������ⶨO2�������
��д��װ��A��B�з�����Ӧ�����ӷ���ʽ��
װ��A�� ��װ��B�� ��
��NaOH�������� ��
�۱�ʵ���в����������ʱӦע���������
��
�������ڶ�����Ͳ��ˮ�������������ɱ�״�������������ΪVmL������Ʒ
�й������Ƶ���������Ϊ ��
��1��ij����������Ʒ�Ѿ����ֱ��ʣ��������ʵ�飬����һ����Һ��֤�����������Ѿ����� ��
��2��������Ʒ�л��й������Ƶķ����� ��
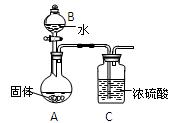
��3��ij����С��Ϊ�˴��Բⶨ�������Ƶ��������������dz�ȡag��Ʒ������
������ͼװ�����ⶨ�������Ƶ�����������
 ��#��#��#Դ��ͼ�е�E��F��������װ�ã������ⶨO2�������
��#��#��#Դ��ͼ�е�E��F��������װ�ã������ⶨO2���������д��װ��A��B�з�����Ӧ�����ӷ���ʽ��
װ��A�� ��װ��B�� ��
��NaOH�������� ��
�۱�ʵ���в����������ʱӦע���������
��
�������ڶ�����Ͳ��ˮ�������������ɱ�״�������������ΪVmL������Ʒ
�й������Ƶ���������Ϊ ��
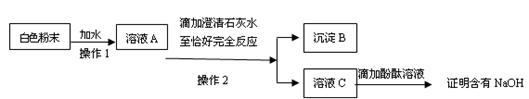
��1��ȡ������Ʒ���ܽ⣬����BaCl2��Һ��������а�ɫ������֤��Na2O2�Ѿ�����(2��)
��2��ȡ������Ʒ�����Թ��У��ټ���������ˮ���Ѵ��л��ǵ�ľ�������Թ��У�ľ����ȼ��֤����Ʒ������Na2O2 (2��)
��3����CaCO3+2H+==Ca2++H2O+CO2��(2��)��
HCO3-+H+==H2O+CO2��(2��)
������δ��Ӧ��CO2(1��)
�۷�Ӧ���ָ�������ʱ�ٶ���������E��F��Һ����ƽ��ƽ�Ӷ�������2�֣�
��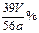 (2��)
(2��)
��2��ȡ������Ʒ�����Թ��У��ټ���������ˮ���Ѵ��л��ǵ�ľ�������Թ��У�ľ����ȼ��֤����Ʒ������Na2O2 (2��)
��3����CaCO3+2H+==Ca2++H2O+CO2��(2��)��
HCO3-+H+==H2O+CO2��(2��)
������δ��Ӧ��CO2(1��)
�۷�Ӧ���ָ�������ʱ�ٶ���������E��F��Һ����ƽ��ƽ�Ӷ�������2�֣�
��
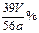 (2��)
(2��)��

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
�����Ŀ
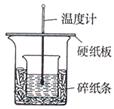
 Ȧ�����ձ�����ֽ���������⣬���貹��IJ��������� _______��
Ȧ�����ձ�����ֽ���������⣬���貹��IJ��������� _______�� ʵ�飬ʵ�������С���һ�¡��ɴ�ȷ�����������ȷ��
ʵ�飬ʵ�������С���һ�¡��ɴ�ȷ�����������ȷ�� ��С�ոĽ��ķ�����_______ ��
��С�ոĽ��ķ�����_______ ��