题目内容
小刚、小军、小红对一久置的NaOH白色粉末的成分进行探究。
三位同学都作出以下的猜想:
Ⅰ.①可能是NaOH; ②可能是Na2CO3; ③可能是NaOH与Na2CO3的混合物。
做出②③猜想的依据是______________________________(用化学方程式表示)
Ⅱ.为了验证①②③猜想,他们分别做了下面的实验。
(1)小刚取少量白色粉末于试管中,再滴加稀HCl,有气泡产生,由此可推出白色粉末中一定含有 ,可肯定猜想________(填序号)是错误的。
(2)小军又取少量白色粉末溶于水,向所得溶液中滴加酚酞试液,溶液变为红色。由此小军结合小刚的实验,认为猜想③是正确的。小红却认为小军的所得结论不确切,因为
。
(3)为探究白色粉末是否含有NaOH,小红设计了如下实验方案: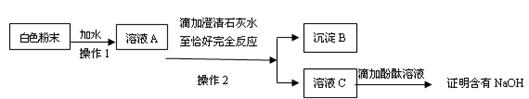
①沉淀B的化学式为____________。
②在操作1中要用到玻璃棒,其作用是_______________;
在操作2中,除要用到铁架台(带铁 圈)、烧杯、滤纸、玻璃棒外,还需补充的玻璃仪器是 _______。
圈)、烧杯、滤纸、玻璃棒外,还需补充的玻璃仪器是 _______。
③小刚认为小红的这个方案也有问题,理由是________________。
Ⅲ.小刚对小红的实验作了正确的改进后进行了 实验,实验现象和小红的一致。由此确定猜想③是正确的
实验,实验现象和小红的一致。由此确定猜想③是正确的 。小刚改进的方法是_______ 。
。小刚改进的方法是_______ 。
三位同学都作出以下的猜想:
Ⅰ.①可能是NaOH; ②可能是Na2CO3; ③可能是NaOH与Na2CO3的混合物。
做出②③猜想的依据是______________________________(用化学方程式表示)
Ⅱ.为了验证①②③猜想,他们分别做了下面的实验。
(1)小刚取少量白色粉末于试管中,再滴加稀HCl,有气泡产生,由此可推出白色粉末中一定含有 ,可肯定猜想________(填序号)是错误的。
(2)小军又取少量白色粉末溶于水,向所得溶液中滴加酚酞试液,溶液变为红色。由此小军结合小刚的实验,认为猜想③是正确的。小红却认为小军的所得结论不确切,因为
。
(3)为探究白色粉末是否含有NaOH,小红设计了如下实验方案:

①沉淀B的化学式为____________。
②在操作1中要用到玻璃棒,其作用是_______________;
在操作2中,除要用到铁架台(带铁
 圈)、烧杯、滤纸、玻璃棒外,还需补充的玻璃仪器是 _______。
圈)、烧杯、滤纸、玻璃棒外,还需补充的玻璃仪器是 _______。③小刚认为小红的这个方案也有问题,理由是________________。
Ⅲ.小刚对小红的实验作了正确的改进后进行了
 实验,实验现象和小红的一致。由此确定猜想③是正确的
实验,实验现象和小红的一致。由此确定猜想③是正确的 。小刚改进的方法是_______ 。
。小刚改进的方法是_______ 。Ⅰ.CO2+2NaOH====Na2CO3+H2O
Ⅱ.(1)Na2CO3 ①
(2)溶液中存在Na2CO3也会使酚酞变红
(3)①CaCO3
②搅拌, 漏斗
③Na2CO3与Ca(OH)2反应后也有NaOH生成,因此这个方案不能证明白色粉末中是否含有NaOH
Ⅲ.改进方案:将滴加澄清石灰水改为过量的CaCl2(或Ba(NO3)2或Ca(NO3)2或BaC l2)
l2)
即可。
Ⅱ.(1)Na2CO3 ①
(2)溶液中存在Na2CO3也会使酚酞变红
(3)①CaCO3
②搅拌, 漏斗
③Na2CO3与Ca(OH)2反应后也有NaOH生成,因此这个方案不能证明白色粉末中是否含有NaOH
Ⅲ.改进方案:将滴加澄清石灰水改为过量的CaCl2(或Ba(NO3)2或Ca(NO3)2或BaC
 l2)
l2)即可。
略

练习册系列答案
相关题目
 是一种大气污染物,某兴趣小组欲探究
是一种大气污染物,某兴趣小组欲探究


 高#考#资#源上图中的E和F构成量气装置,用来测定O2的体积。
高#考#资#源上图中的E和F构成量气装置,用来测定O2的体积。
 .
.