��Ŀ����
��2009?�Ͼ���ģ��ij��θҩ��ֹ���ΪCaCO3��Ϊ�ⶨ����CaCO3������ij�о�С������������ַ�����ҩƬ�е������ɷ���HCl��NaOH��Һ������Ӧ������ش��й����⣺
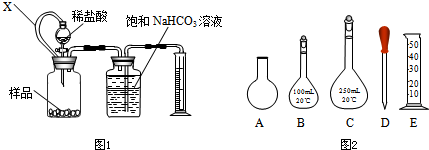
��1������һ������ͼ1װ�ò���һ������Ʒ������ϡ���ᷴӦ����CO2�����������CaCO3�ĺ�����װ������ͨ��X��������
��2�����������õζ����ⶨ�������¼���������
A������0.100mol/L HCl��Һ��0.100mol/L NaOH��Һ
B��ȡһ��ҩƬ��0.100g������������20.0mL����ˮ
C���Է�̪Ϊָʾ������0.100mol/L NaOH��Һ�ζ�����ȥ���ΪVmL�ﵽ�յ�
D������ʽ�ζ��ܼ���25.00mL 0.100mol/L HCl��Һ����ַ�Ӧ
�ٲⶨ���̵���ȷ����˳��Ϊ
������Ҫ�ظ��ζ�4�Σ�����0.1mol/L HCl��Һ��Ҫѡ��ͼ2��ʾ�����е�
�۾��ⶨ��ÿ������NaOH��Һ�����ƽ��ֵΪ13.00mL�����θҩ��CaCO3����������
���뷽��һ��ȣ�����������Ҫ�ŵ���

��1������һ������ͼ1װ�ò���һ������Ʒ������ϡ���ᷴӦ����CO2�����������CaCO3�ĺ�����װ������ͨ��X��������
ʹ���ƿ���Һ©��������ѹǿ��ͬ�����ڵ�����Һ
ʹ���ƿ���Һ©��������ѹǿ��ͬ�����ڵ�����Һ
��������������������������������Ӱ��
������������������������������Ӱ��
����2�����������õζ����ⶨ�������¼���������
A������0.100mol/L HCl��Һ��0.100mol/L NaOH��Һ
B��ȡһ��ҩƬ��0.100g������������20.0mL����ˮ
C���Է�̪Ϊָʾ������0.100mol/L NaOH��Һ�ζ�����ȥ���ΪVmL�ﵽ�յ�
D������ʽ�ζ��ܼ���25.00mL 0.100mol/L HCl��Һ����ַ�Ӧ
�ٲⶨ���̵���ȷ����˳��Ϊ
ABDC ��BADC
ABDC ��BADC
������ĸ����������Ҫ�ظ��ζ�4�Σ�����0.1mol/L HCl��Һ��Ҫѡ��ͼ2��ʾ�����е�
CD��CDE
CD��CDE
������ĸ�����۾��ⶨ��ÿ������NaOH��Һ�����ƽ��ֵΪ13.00mL�����θҩ��CaCO3����������
60%
60%
�����뷽��һ��ȣ�����������Ҫ�ŵ���
�ζ���������Ʒ���Լ����٣�ʵ��ⶨ������С
�ζ���������Ʒ���Լ����٣�ʵ��ⶨ������С
����������1������ʵ��һ�����ܱջ����½��У�����һ��Һ���ᷢ������ѹ��ƽ���������ʱҺ��Ͳ����£�������ͨ�ܿ�ƽ������ѹ����Һ��˳�����£���ʵ����ͨ������CO2������CaCO3������������������Ƿ�������ʵ��ijɹ�������ڵ���Һ��ʱ�����ƿ��ԭ�е�����Ҳ����Ӧ���뼯��ƿ�У�������ʹCO2��ƫ������ͨ�ܿ�ʹ�ⲿ������ص���Һ©���У������뼯��ƿ�У���Сʵ����
��2��������A��B�Dz��в������������Ⱥ����⣮
����Ϊÿ�εζ��õ�������25.00 mL���ظ�����4 �Σ��ټ�����ϴ�ζ��ܡ����㡢������Ҳ���������ᣬ�������������100 mL��������ʱ����250 mL������ƿ������ʱ��Ҫ�ý�ͷ�ιܣ�����ˮϡ��ʱҲ���Խ�����Ͳ����ˮ������
�۷�Ӧ��������������������Һ��Ũ����ȣ�����������Һ�����Ϊ13.00 mL������̼��Ʒ�Ӧ������Ϊ25.00mL-13.00 mL=12.00 mL�����ݷ�Ӧ��CaCO3+2HCl=CaCl2+H2O+CO2������̼��Ƶ��������ٸ�����������������㣮
���뷽��һ��ȣ����������ŵ��ǵζ���������Ʒ���Լ����٣�ʵ��ⶨ������С��
��2��������A��B�Dz��в������������Ⱥ����⣮
����Ϊÿ�εζ��õ�������25.00 mL���ظ�����4 �Σ��ټ�����ϴ�ζ��ܡ����㡢������Ҳ���������ᣬ�������������100 mL��������ʱ����250 mL������ƿ������ʱ��Ҫ�ý�ͷ�ιܣ�����ˮϡ��ʱҲ���Խ�����Ͳ����ˮ������
�۷�Ӧ��������������������Һ��Ũ����ȣ�����������Һ�����Ϊ13.00 mL������̼��Ʒ�Ӧ������Ϊ25.00mL-13.00 mL=12.00 mL�����ݷ�Ӧ��CaCO3+2HCl=CaCl2+H2O+CO2������̼��Ƶ��������ٸ�����������������㣮
���뷽��һ��ȣ����������ŵ��ǵζ���������Ʒ���Լ����٣�ʵ��ⶨ������С��
����⣺��1������ʵ��һ�����ܱջ����½��У�����һ��Һ���ᷢ������ѹ��ƽ���������ʱҺ��Ͳ����£�������ͨ�ܿ�ƽ������ѹ����Һ��˳�����£���ʵ����ͨ������CO2������CaCO3������������������Ƿ�������ʵ��ijɹ�������ڵ���Һ��ʱ�����ƿ��ԭ�е�����Ҳ����Ӧ���뼯��ƿ�У�������ʹCO2��ƫ������ͨ�ܿ�ʹ�ⲿ������ص���Һ©���У������뼯��ƿ�У���Сʵ����
�ʴ�Ϊ��ʹ���ƿ���Һ©��������ѹǿ��ͬ�����ڵ�����Һ�� ������������������������������Ӱ�죮
��2��������A��B�Dz��в������������Ⱥ����⣬�ʿ�����ABDC ��BADC��
�ʴ�Ϊ��ABDC ��BADC��
����Ϊÿ�εζ��õ�������25.00 mL���ظ�����4 �Σ��ټ�����ϴ�ζ��ܡ����㡢������Ҳ���������ᣬ�������������100 mL��������ʱ����250 mL������ƿ������ʱ��Ҫ�ý�ͷ�ιܣ�����ˮϡ��ʱҲ���Խ�����Ͳ����ˮ������
��ѡ��CD��CDE��
�۷�Ӧ��������������������Һ��Ũ����ȣ�����������Һ�����Ϊ13.00 mL������̼��Ʒ�Ӧ������Ϊ25.00mL-13.00 mL=12.00 mL�����ݷ�Ӧ��CaCO3+2HCl=CaCl2+H2O+CO2����֪��100g��2mol=m��CaCO3����0.100 mol/L��0.012L����ã�m=0.06 g��̼��Ƶ���������Ϊ
��100%=60%��
�ʴ�Ϊ��60%��
���뷽��һ��ȣ����������ŵ��ǵζ���������Ʒ���Լ����٣�ʵ��ⶨ������С��
�ʴ�Ϊ���ζ���������Ʒ���Լ����٣�ʵ��ⶨ������С��
�ʴ�Ϊ��ʹ���ƿ���Һ©��������ѹǿ��ͬ�����ڵ�����Һ�� ������������������������������Ӱ�죮
��2��������A��B�Dz��в������������Ⱥ����⣬�ʿ�����ABDC ��BADC��
�ʴ�Ϊ��ABDC ��BADC��
����Ϊÿ�εζ��õ�������25.00 mL���ظ�����4 �Σ��ټ�����ϴ�ζ��ܡ����㡢������Ҳ���������ᣬ�������������100 mL��������ʱ����250 mL������ƿ������ʱ��Ҫ�ý�ͷ�ιܣ�����ˮϡ��ʱҲ���Խ�����Ͳ����ˮ������
��ѡ��CD��CDE��
�۷�Ӧ��������������������Һ��Ũ����ȣ�����������Һ�����Ϊ13.00 mL������̼��Ʒ�Ӧ������Ϊ25.00mL-13.00 mL=12.00 mL�����ݷ�Ӧ��CaCO3+2HCl=CaCl2+H2O+CO2����֪��100g��2mol=m��CaCO3����0.100 mol/L��0.012L����ã�m=0.06 g��̼��Ƶ���������Ϊ
| 0.06g |
| 0.1g |
�ʴ�Ϊ��60%��
���뷽��һ��ȣ����������ŵ��ǵζ���������Ʒ���Լ����٣�ʵ��ⶨ������С��
�ʴ�Ϊ���ζ���������Ʒ���Լ����٣�ʵ��ⶨ������С��
���������⿼���ʵ��ԭ����������ʵ��������ۡ����ʺ����ⶨ���ζ�ԭ�����ã���ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ѧϰ��ȫ����ջ���֪ʶ��

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ
 ����˵��������ǣ�������
����˵��������ǣ�������