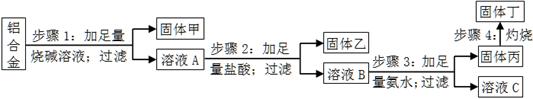
��Ŀ����
ij���Ͻ��к��е�������þ��ͭ���裬Ϊ�˲ⶨ�úϽ������ĺ��������������ʵ�����̣���ش��й����⣺
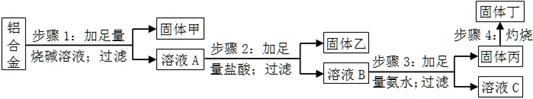
��1������1���ù���ijɷ�Ϊ ��
��2������2�м��������ᣬ��������ͨ������������̼��ԭ��Ϊ ��
��3������3�����ɹ���������ӷ���ʽΪ ����ҺC�������е������ӷ���Ϊ ��
��4������4��������Ӧ�Ļ�ѧ����ʽΪ ��
��5������������ƽ��ȡ10.0g���Ͻ������������ͼ��ʾ���������ù��嶡����Ϊ15.3g��������Ͻ���������������Ϊ �����ж����²����������������ⶨ��Ӱ�죨�ƫ����ƫС������Ӱ�족����
a������1���ռ������㣬�� ��
b������4�����ղ���֣��� ��
(16��)
��1��þ��ͭ��2�֣�
��2��ͨ�������̼�õ����������������Ĺ�����������衢��Ԫ�ط��뿪������2�֣�
��3��Al3+ + 3NH3��H2O = Al(OH)3��+ 3NH4+��3�֣���Na+��NH4+��2�֣�
��4��2Al(OH)3 Al2O3 + 3H2O��3�֣�
Al2O3 + 3H2O��3�֣�
��5��81.0%��2�֣���ƫС��1�֣���ƫ��1�֣�
��������
�����������1��þ��ͭ�����ռ���Һ��Ӧ���ʹ���ijɷ�Ϊþ��ͭ��
��2�����������������Ժ����ᷴӦ�������ܺͶ�����̼��Ӧ���ʲ�����ͨ������������̼��ԭ����ͨ�������̼�õ����������������Ĺ�����������衢��Ԫ�ط��뿪����
��3�������������������백ˮ��Ӧ���ʲ���3�����ɹ���������ӷ���ʽΪAl3+ + 3NH3��H2O = Al(OH)3��+ 3NH4+����ҺC�������е������ӷ���ΪNa+��NH4+��
��4�����������������ʷ�Ӧ����ʽΪ2Al(OH)3 Al2O3 + 3H2O��
Al2O3 + 3H2O��
��5��Al��1/2Al2O3
27g 51g
m 15.3g
51g��m=27g��15.3g
���m=8.1g
��10.0g���Ͻ���������������Ϊ81.0%��
a������1���ռ������㣬Al��Ӧ���꣬��ƫС��
b������1�����ղ���֣�����������ƫ�������������ⶨҪƫ��
���㣺̽�����ʵ���ɻ�������ʵĺ��� ���Ļ�ѧ����
���������⿼��ѧ����ʵ�������ԭ�������ʵķ����ᴿ�����ʺ����IJⶨ����ѧ����ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���������֪ʶ���������������������

| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
��2����������Al�������ȷ�Ӧ����
A��MnO2 B��Na2O C��MgO D��FeO
��3�����ϱ����ݸ�ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ���֤���������õĿ�״�������к��н������������Լ���
��ijͬѧΪ����֤�����к��е⣬���������ʵ�飬��ش�������⣮
��1����1�������գ������ǽ������������ճɻҽ����ù����н�ʹ�õ���ʵ��������
A���Թ� B������ C���ձ� D�����Ǽ�
E�������� F�������� G������̨ H����Ͳ
��2����2����I����Һ�Ļ�ȡ�������ǽ��ҽ�ת�Ƶ��ձ��У�����������ˮ���ò�����ֽ��裬��У���ȴ��
��3����3����������ȡ��2������Һ�������μ�����ʵ��Լ����������������ѡ
A��Ũ���� B��������ˮ C��KMnO4��Һ D��H2O2
��4����4�����ⵥ�ʵļ��飮������ȡ������3������Һ���μ�
��ijͬѧ�������ȷ�Ӧ����ʵ�顣���ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | ���� |
��������Al�������ȷ�Ӧ���� ��������ţ�
A��MnO2 B��Na2O C��MgO D��FeO
���ϱ����ݸ�ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ���֤���������õĿ�״�������к��н������������Լ��� �����Լ����ʵĵ���ʽΪ ��
��ijͬѧΪ����֤�����к��е⣬���������ʵ�飬��ش�������⡣
��1����1�������ա������ǽ������������ճɻҽ����ù����н�ʹ�õ���ʵ��������
_______________�ȡ�������ţ���ͬ��
A���Թܣ�B��������C���ձ���D�����Ǽܣ�E�������ǣ�F�������ƣ�G������̨��H����Ͳ
��2����I����Һ�Ļ�ȡ�������ǽ��ҽ�ת�Ƶ��ձ��У�����������ˮ���ò�����ֽ��裬��У���ȴ��________��������뷽����
��3����3����������ȡ(2)����Һ�������μ�����ʵ��Լ����������������ѡ_________��
A��Ũ���� B��������ˮ C��KMnO4��Һ D��H2O2
��4����4�����ⵥ�ʵļ��顣������ȡ������3������Һ���μ�_______��Һ��֤�������к��⡣
��ijͬѧ�������ȷ�Ӧ����ʵ�顣���ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
|
���� |
Al |
Al2O3 |
Fe |
Fe2O3 |
|
�۵�/�� |
660 |
2054 |
1535 |
1462 |
|
�е�/�� |
2467 |
2980 |
2750 |
���� |
���ȷ�Ӧ����ʽΪ .
��������Al�������ȷ�Ӧ���� ��������ţ�
A��MnO2 B��Na2O C��MgO D��FeO
���ϱ����ݸ�ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ���֤���������õĿ�״�������к��н������������Լ��� �����Լ����ʵĵ���ʽΪ ��
��ijͬѧΪ����֤�����к��е⣬���������ʵ�飬��ش�������⡣
��1����1�������ա������ǽ������������ճɻҽ����ù����н�ʹ�õ���ʵ��������
_______________�ȡ�������ţ���ͬ��
A���Թܣ�B��������C���ձ���D�����Ǽܣ�E�������ǣ�F�������ƣ�G������̨��H����Ͳ
��2����I����Һ�Ļ�ȡ�������ǽ��ҽ�ת�Ƶ��ձ��У�����������ˮ���ò�����ֽ��裬��У���ȴ��________��������뷽����
��3����3����������ȡ(2)����Һ�������μ�����ʵ��Լ����������������ѡ_________��
A��Ũ���� B��������ˮ C��KMnO4��Һ D��H2O2
��4����4�����ⵥ�ʵļ��顣������ȡ������3������Һ���μ�_______��Һ��֤�������к��⡣