��Ŀ����
��ijͬѧ�������ȷ�Ӧ����ʵ�顣���ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | ���� |
��������Al�������ȷ�Ӧ���� ��������ţ�
A��MnO2 B��Na2O C��MgO D��FeO
���ϱ����ݸ�ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ���֤���������õĿ�״�������к��н������������Լ��� �����Լ����ʵĵ���ʽΪ ��
��ijͬѧΪ����֤�����к��е⣬���������ʵ�飬��ش�������⡣
��1����1�������ա������ǽ������������ճɻҽ����ù����н�ʹ�õ���ʵ��������
_______________�ȡ�������ţ���ͬ��
A���Թܣ�B��������C���ձ���D�����Ǽܣ�E�������ǣ�F�������ƣ�G������̨��H����Ͳ
��2����I����Һ�Ļ�ȡ�������ǽ��ҽ�ת�Ƶ��ձ��У�����������ˮ���ò�����ֽ��裬��У���ȴ��________��������뷽����
��3����3����������ȡ(2)����Һ�������μ�����ʵ��Լ����������������ѡ_________��
A��Ũ���� B��������ˮ C��KMnO4��Һ D��H2O2
��4����4�����ⵥ�ʵļ��顣������ȡ������3������Һ���μ�_______��Һ��֤�������к��⡣
��1��2Al+Fe2O3 2Fe+Al2O3 ��1�֣�
2Fe+Al2O3 ��1�֣�
��2��A D ��2�֣� ��3������������Һ��2�֣���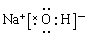 ��1�֣�
��1�֣�
��1��BDEF��2�֣���2������ ��2�֣� ��3��D��1�֣� ��4) ���ۣ�1�֣�
���������������1���ڸ����£��������ܺ������������û���Ӧ���������ȷ�Ӧ����ʽΪ2Al+Fe2O3 2Fe+Al2O3��
2Fe+Al2O3��
��2���ƺ�þ�ǻ��õĽ������������ƺ�����þ���������������ȷ�Ӧ���������̺������������ԣ���ѡAD��
��3���������������������У������������ܺ�����������Һ��Ӧ��������֤���������õĿ�״�������к��н������������Լ�������������Һ���������������ӻ���������ʽ��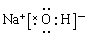 ��
��
��1�����������������н��У�����������Ҫ���������ǵ����ż��ϣ�������Ҫ�������������������ǡ����żܺ� �ƾ��ƣ�����ѡBDEF��
��2�����������ԵĹ���Ϳ����Եĺ��е�Ԫ�ص�Һ��IJ����ǹ��ˡ�
��3�����������Ե����ʿ��Խ�����������Ϊ���ʵ⣬˫������ɫ�������������Ի����£����������ӵ�ʵ���ǣ�H2O2+2H++2I����2H2O+I2����ԭ������ˮ������������ʣ����Դ�ѡD��
��4��������������ɫ����μӵ�����Һ��֤�������к��⡣
���㣺�������ȷ�Ӧ�������е���ȡ���й��ж��Լ��йػ���ʵ�����
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ��ʵ��������������ѵ������ѧʵ�鳣��������ʹ�÷����ͻ�ѧʵ����������ǽ��л�ѧʵ��Ļ������Ի�ѧʵ��Ŀ����벻����ѧʵ��Ļ������������Ա����������ڸ߿�������һ�����ǵ������⣬��һ�����dz������ۺ�ʵ�������У���ǰ����˵�������Ȼ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | --- |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���
��3��ʵ�����ܽ������������Լ�����õ���
A��Ũ���� B��ϡ���� ������C��ϡ���� ������D������������Һ

��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�Ϊ�˲ⶨ�����������ֽ��������ʵ���֮�ȣ�ijͬѧȡһ������������������������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol/L������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
��1����μ���4mol/L������������Һ�Ķ�����������
��2��������ͼ�η�����֪����Һ�н��OH-������ǿ��������
��3��ͨ����ͼ�����Լ��㣬�����������ֽ��������ʵ���֮��n��Fe����n��Al��=
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
��2����������Al�������ȷ�Ӧ����
A��MnO2 B��Na2O C��MgO D��FeO
��3�����ϱ����ݸ�ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ���֤���������õĿ�״�������к��н������������Լ���
��ijͬѧΪ����֤�����к��е⣬���������ʵ�飬��ش�������⣮
��1����1�������գ������ǽ������������ճɻҽ����ù����н�ʹ�õ���ʵ��������
A���Թ� B������ C���ձ� D�����Ǽ�
E�������� F�������� G������̨ H����Ͳ
��2����2����I����Һ�Ļ�ȡ�������ǽ��ҽ�ת�Ƶ��ձ��У�����������ˮ���ò�����ֽ��裬��У���ȴ��
��3����3����������ȡ��2������Һ�������μ�����ʵ��Լ����������������ѡ
A��Ũ���� B��������ˮ C��KMnO4��Һ D��H2O2
��4����4�����ⵥ�ʵļ��飮������ȡ������3������Һ���μ�
��ijͬѧ�������ȷ�Ӧ����ʵ�顣���ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
|
���� |
Al |
Al2O3 |
Fe |
Fe2O3 |
|
�۵�/�� |
660 |
2054 |
1535 |
1462 |
|
�е�/�� |
2467 |
2980 |
2750 |
���� |
���ȷ�Ӧ����ʽΪ .
��������Al�������ȷ�Ӧ���� ��������ţ�
A��MnO2 B��Na2O C��MgO D��FeO
���ϱ����ݸ�ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ���֤���������õĿ�״�������к��н������������Լ��� �����Լ����ʵĵ���ʽΪ ��
��ijͬѧΪ����֤�����к��е⣬���������ʵ�飬��ش�������⡣
��1����1�������ա������ǽ������������ճɻҽ����ù����н�ʹ�õ���ʵ��������
_______________�ȡ�������ţ���ͬ��
A���Թܣ�B��������C���ձ���D�����Ǽܣ�E�������ǣ�F�������ƣ�G������̨��H����Ͳ
��2����I����Һ�Ļ�ȡ�������ǽ��ҽ�ת�Ƶ��ձ��У�����������ˮ���ò�����ֽ��裬��У���ȴ��________��������뷽����
��3����3����������ȡ(2)����Һ�������μ�����ʵ��Լ����������������ѡ_________��
A��Ũ���� B��������ˮ C��KMnO4��Һ D��H2O2
��4����4�����ⵥ�ʵļ��顣������ȡ������3������Һ���μ�_______��Һ��֤�������к��⡣
��6�֣�ijͬѧ�������ȷ�Ӧ����ʵ�顣���ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3
�۵㡢�е��������£�
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | ���� |
��1�����ȷ�Ӧ����ʽΪ .
��2����������Al�������ȷ�Ӧ���� ��������ţ�
A��MnO2 B��Na2O C��MgO D��FeO
��3�����ϱ����ݸ�ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ���֤���������õĿ�
״�������к��н������������Լ��� �����Լ����ʵĵ���ʽΪ ��
��(6��)ijͬѧΪ����֤�����к��е⣬���������ʵ�飬��ش�������⡣
��1����1�������ա������ǽ������������ճɻҽ����ù����н�ʹ�õ���ʵ��������
_______________�ȡ�������ţ���ͬ��
A���Թ� B������ C���ձ� D�����Ǽ�
E�������� F�������� G������̨ H����Ͳ
��2����2����I����Һ�Ļ�ȡ�������ǽ��ҽ�ת�Ƶ��ձ��У�����������ˮ���ò������
���裬��У���ȴ��________��������뷽����
��3����3����������ȡ(2)����Һ�������μ�����ʵ��Լ����������������ѡ_________��
A��Ũ���� B��������ˮ C��KMnO4��Һ D��H2O2
��4����4�����ⵥ�ʵļ��顣������ȡ������3������Һ���μ�_______��Һ��֤����
���к��⡣