��Ŀ����
(9��)�����ڵ���������Ԫ��A��B��C��D��Eԭ���������α�С��A�ĵ����ڳ�����Ϊ��̬��B��D����ͬһ���壬CΪ��Ȼ�纬�����Ľ���Ԫ�أ�D������������Ϊ������2����EΪ���ڱ���ԭ�Ӱ뾶��С��Ԫ�ء�
(1)A��B��Ԫ�����Ʒֱ�Ϊ �� ��
(2)D��E�����γɶ��ֻ��������һ�ֻ��������Է�������Ϊ28��д����ṹ��
ʽ ��
(3)��Ȼ����BԪ�ض������������ʽ���ڣ���д����ҵ����ȡB���ʵĻ�ѧ����ʽ
��
(4)д��ʵ������ȡA����Ļ�ѧ����ʽ ��
(5)a��C���ʺ��������Ļ�����Ϊ���ȷݣ�������һ���м�������NaOH��Һ�������������(�������ͬ)���ΪmL����һ���ڸ�����ǡ����ȫ��Ӧ����������ȴ���������HCl��Һ����������������ΪnL����m��n= ��
(1)A��B��Ԫ�����Ʒֱ�Ϊ �� ��
(2)D��E�����γɶ��ֻ��������һ�ֻ��������Է�������Ϊ28��д����ṹ��
ʽ ��
(3)��Ȼ����BԪ�ض������������ʽ���ڣ���д����ҵ����ȡB���ʵĻ�ѧ����ʽ
��
(4)д��ʵ������ȡA����Ļ�ѧ����ʽ ��
(5)a��C���ʺ��������Ļ�����Ϊ���ȷݣ�������һ���м�������NaOH��Һ�������������(�������ͬ)���ΪmL����һ���ڸ�����ǡ����ȫ��Ӧ����������ȴ���������HCl��Һ����������������ΪnL����m��n= ��
��1����2����ÿ��1�֣�����ÿ��2��
��1���� ���� ��2�� CH2��CH2��3��SiO2��2C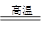 Si��2CO��
Si��2CO��
��4�� 4HCl(Ũ)��MnO2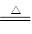 MnCl2��2H2O��Cl2�� ��5��3:2
MnCl2��2H2O��Cl2�� ��5��3:2
��1���� ���� ��2�� CH2��CH2��3��SiO2��2C
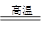 Si��2CO��
Si��2CO�� ��4�� 4HCl(Ũ)��MnO2
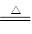 MnCl2��2H2O��Cl2�� ��5��3:2
MnCl2��2H2O��Cl2�� ��5��3:2��Ȼ�纬�����Ľ���Ԫ����������C��Al������������Ϊ������2����Ԫ��ֻ����C����D��̼Ԫ�ء�B��D����ͬһ���壬����B��Si��A��ԭ����������Si�ģ���A�ĵ����ڳ�����Ϊ��̬������A��Cl��E��ԭ��������С����EΪ���ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�����E����Ԫ�ء�
��1����
��2����̼�����γɵĻ���������Է�������Ϊ28����ϩ����ϩ����̼̼˫������ṹ��ʽΪCH2��CH2��
��3����ҵ����ȡ���ʹ�ķ������ڸ������ý�̿��ԭ�������裬��ӦʽΪSiO2��2C Si��2CO����
Si��2CO����
��4��ʵ������ȡ�����ķ�ӦʽΪ4HCl(Ũ)��MnO2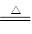 MnCl2��2H2O��Cl2����
MnCl2��2H2O��Cl2����
��5�������������Ļ������뵽����������Һ�У�ֻ�������������Ʒ�Ӧ�������������������ʵ�����1mol������������1.5mol�����ڸ����·�Ӧ���������ȷ�Ӧ�����ɵ������������ʵ�����1mol������������������ᷴӦ�����Ȼ�������1mol������������������֮����3:2��
��1����
��2����̼�����γɵĻ���������Է�������Ϊ28����ϩ����ϩ����̼̼˫������ṹ��ʽΪCH2��CH2��
��3����ҵ����ȡ���ʹ�ķ������ڸ������ý�̿��ԭ�������裬��ӦʽΪSiO2��2C
 Si��2CO����
Si��2CO������4��ʵ������ȡ�����ķ�ӦʽΪ4HCl(Ũ)��MnO2
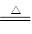 MnCl2��2H2O��Cl2����
MnCl2��2H2O��Cl2������5�������������Ļ������뵽����������Һ�У�ֻ�������������Ʒ�Ӧ�������������������ʵ�����1mol������������1.5mol�����ڸ����·�Ӧ���������ȷ�Ӧ�����ɵ������������ʵ�����1mol������������������ᷴӦ�����Ȼ�������1mol������������������֮����3:2��

��ϰ��ϵ�д�
�����Ŀ
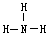
 ��NH
��NH